Bernoulli probit regression with selected constrained species
Source:vignettes/jSDM_binomial_probit_sp_constrained.Rmd
jSDM_binomial_probit_sp_constrained.Rmd1 Load librairies
# ===================================================
# Load libraries
# ===================================================
library(ggplot2)
library(coda)
library(parallel)
library(doParallel)
#> Loading required package: foreach
#> Loading required package: iterators
library(jSDM)
#> ##
#> ## jSDM R package
#> ## For joint species distribution models
#> ## https://ecology.ghislainv.fr/jSDM
#> ##
library(kableExtra)2 Model definition
Referring to the models used in the articles Warton et al. (2015) and Albert & Siddhartha (1993), we define the following model :
\[ \mathrm{probit}(\theta_{ij}) =\alpha_i + X_i.\beta_j+ W_i.\lambda_j \]
Considering the latent variable \(z\) such as \(z_{ij} = \alpha_i + \beta_{0j} + X_i.\beta_j + W_i.\lambda_j + \epsilon_{i,j}\), with \(\forall (i,j) \ \epsilon_{ij} \sim \mathcal{N}(0,1)\),
it can be easily shown that: \(y_{ij} \sim \mathcal{B}ernoulli(\theta_{ij})\), with:
Link function probit: \(\mathrm{probit}: q \rightarrow \Phi^{-1}(q)\) where \(\Phi\) correspond to the distribution function of the reduced centered normal distribution.
\(\theta_{ij}\): occurrence probability of the species \(j\) on site \(i\) for \(j=1,\ldots,J\) and for \(i=1,\ldots,I\).
Response variable: \(Y=(y_{ij})^{i=1,\ldots,nsite}_{j=1,\ldots,nsp}\) such as:
\[y_{ij}=\begin{cases} 0 & \text{ if species $j$ is absent on the site $i$}\\ 1 & \text{ if species $j$ is present on the site $i$}. \end{cases}\]
\(X_i\): Vector of explanatory variables for site \(i\) with \(X_i=(x_i^1,\ldots,x_i^p)\in \mathbb{R}^p\) where \(p\) is the number of bio-climatic variables considered (including intercept \(\forall i, x_i^1=1\)).
\(\alpha_i\): Random effect of site \(i\) such as \(\alpha_i \sim \mathcal{N}(0, V_{\alpha})\), corresponds to a mean suitability for site \(i\). We assumed that \(V_{\alpha} \sim \mathcal{IG}(\text{shape}=0.1, \text{rate}=0.1)\).
\(\beta_j\): Effects of the explanatory variables on the probability of presence of species \(j\) including species intercept (\(\beta_{0j}\)). We use a prior distribution \(\beta_j \sim \mathcal{N}_p(0,1)\).
\(W_i\): Vector of random latent variables for site \(i\) such as \(W_i \sim \mathcal{N}_q(0, 1)\) where the number of latent variables \(q\) must be fixed by the user (default to \(q=2\)).
\(\lambda_j\): Effects of the latent variables on the probability of presence of species \(j\) also known as “factor loadings” (Warton et al. 2015). We use the following prior distribution to constraint values to \(0\) on upper diagonal and to strictly positive values on diagonal, for \(j=1,\ldots,J\) and \(l=1,\ldots,q\) : \[\lambda_{jl} \sim \begin{cases} \mathcal{N}(0,1) & \text{if } l < j \\ \mathcal{N}(0,1) \text{ left truncated by } 0 & \text{if } l=j \\ P \text{ such as } \mathbb{P}(\lambda_{jl} = 0)=1 & \text{if } l>j \end{cases}\].
This model is equivalent to a multivariate GLMM \(\mathrm{g}(\theta_{ij}) = X_i.\beta_j + u_{ij}\), where \(u_{ij} \sim \mathcal{N}(0, \Sigma)\) with the constraint that the variance-covariance matrix \(\Sigma = \Lambda \Lambda^{\prime}\), where \(\Lambda\) is the full matrix of factor loadings, with the \(\lambda_j\) as its columns.
3 Occurrence data-set

Figure 3.1: Fungi (Wilkinson et al. 2019).
This data-set is available in the jSDM-package R package. It can be loaded with the data() command. The fungi dataset is in “wide” format: each line is a site and the occurrence data are in columns. A site is characterized by its x-y geographical coordinates twelve covariates.
library(jSDM)
# frogs data
data(fungi, package="jSDM")
head(fungi)
#> antser antsin astfer fompin hetpar junlut phefer phenig phevit poscae triabi
#> 1 0 0 0 0 0 0 1 0 0 0 0
#> 2 0 0 0 0 0 0 0 0 0 0 0
#> 3 0 0 0 0 0 0 0 0 1 0 0
#> 4 0 0 0 0 0 0 0 0 1 0 0
#> 5 0 0 0 0 0 0 0 0 0 0 0
#> 6 0 0 0 0 0 0 0 0 0 0 0
#> diam dc1 dc2 dc3 dc4 dc5 quality3 quality4 ground3 ground4 epi
#> 1 1.3699426 1 0 0 0 0 0 1 0 1 -0.6626584
#> 2 -0.2083835 0 0 0 0 0 1 0 0 0 -0.6626584
#> 3 0.2435434 0 0 0 0 0 0 1 0 1 -0.3568567
#> 4 0.8040889 1 0 0 0 0 0 1 0 0 -0.6626584
#> 5 -0.5927520 0 0 0 0 0 0 1 0 0 -0.6626584
#> 6 -0.8227620 0 0 1 0 0 0 0 0 1 -0.3568567
#> bark
#> 1 0.5297272
#> 2 1.2511949
#> 3 -1.0334527
#> 4 -0.9132081
#> 5 1.2511949
#> 6 1.2511949We rearrange the data in two data-sets: a first one for the presence-absence observations for each species (columns) at each site (rows), and a second one for the site characteristics.
We also normalize the continuous explanatory variables to facilitate MCMC convergence.
# Fungi data-set
data(fungi, package="jSDM")
PA_Fungi <- fungi[,c("antser","antsin","astfer","fompin","hetpar","junlut",
"phefer","phenig","phevit","poscae","triabi")]
# Normalized continuous variables
Env_Fungi <- cbind(scale(fungi[,c("diam","epi","bark")]),
fungi[,c("dc1","dc2","dc3","dc4","dc5",
"quality3","quality4","ground3","ground4")])
colnames(Env_Fungi) <- c("diam","epi","bark","dc1","dc2","dc3","dc4","dc5",
"quality3","quality4","ground3","ground4")
# Remove sites where none species was recorded
Env_Fungi <- Env_Fungi[rowSums(PA_Fungi) != 0,]
PA_Fungi<- PA_Fungi[rowSums(PA_Fungi) != 0,]4 Fitting JSDM with the species on first columns constrained
4.1 Parameter inference
As a first step, we use the jSDM_binomial_probit() function to fit the JSDM in parallel, to obtain four MCMC chains of parameters, with the constrained species arbitrarily chosen as the first ones in the presence-absence data-set.
## Make a cluster for parallel MCMCs
nchains <- 4
ncores <- nchains ## One core for each MCMC chains
clust <- makeCluster(ncores)
registerDoParallel(clust)
# Seeds
seed_mcmc <- c(1, 12, 123, 1234)
# Model
mod_Fungi <-
foreach (i = 1:nchains) %dopar% {
# Inferring model parameters
mod <- jSDM::jSDM_binomial_probit(# Chains
burnin=5000,
mcmc=10000,
thin=10,
# Response variable
presence_data = PA_Fungi,
# Explanatory variables
site_formula = ~.,
site_data = Env_Fungi,
# Model specification
n_latent=2,
site_effect="random",
# Starting values
alpha_start=0,
beta_start=0,
lambda_start=0,
W_start=0,
V_alpha=1,
# Priors
shape=0.1,
rate=0.1,
mu_beta=0,
V_beta=1,
mu_lambda=0,
V_lambda=1,
# Other
seed = seed_mcmc[i],
verbose = 0
)
return(mod)
}
# Stop cluster
stopCluster(clust)4.2 The Gelman–Rubin convergence diagnostic
4.2.1 Definition
The Gelman–Rubin diagnostic evaluates MCMC convergence by analyzing the difference between multiple Markov chains. The convergence is assessed by comparing the estimated between-chains and within-chain variances for each model parameter. Large differences between these variances indicate nonconvergence. See Gelman & Rubin (1992) and Brooks & Gelman (1998) for the detailed description of the method.
Suppose we have \(M\) chains, each of length \(N\), although the chains may be of different lengths. The same-length assumption simplifies the formulas and is used for convenience. For a model parameter \(\theta\), let \(\left(\theta_{mt}\right)_{t=1}^N\) be the \(m\)th simulated chain, \(m=1,\dots,M\). Let \(\hat{\theta}_m=\frac{1}{N}\sum\limits_{t=1}^N \hat{\theta}_{mt}\) and \(\hat{\sigma}^2_m=\frac{1}{N-1}\sum\limits_{t=1}^N (\hat{\theta}_{mt}-\hat{\theta}_m)^2\) be the sample posterior mean and variance of the \(m\)th chain, and let the overall sample posterior mean be \(\hat{\theta}=\frac{1}{M}\sum\limits_{m=1}^M \hat{\theta}_m\).
The between-chains and within-chain variances are given by \[B=\frac{N}{M-1}\sum\limits_{m=1}^M (\hat{\theta}_m - \hat{\theta})^2\] \[W=\frac{1}{M}\sum\limits_{m=1}^M\hat{\sigma}^2_m\]
Under certain stationarity conditions, the pooled variance :
\[\hat{V}=\frac{N-1}{N}W + \frac{M+1}{MN}B\]
is an unbiased estimator of the marginal posterior variance of \(\theta\) (Gelman & Rubin (1992)). The potential scale reduction factor (PSRF) is defined to be the ratio of \(\hat{V}\) and \(W\). If the \(M\) chains have converged to the target posterior distribution, then PSRF should be close to 1. The article Brooks & Gelman (1998) corrected the original PSRF by accounting for sampling variability as follows: \[ \hat{R}= \sqrt{\frac{\hat{d}+3}{\hat{d}+1}\frac{\hat{V}}{W}}\]
where \(\hat{d}\) is the degrees of freedom estimate of a \(t\) distribution.
PSRF estimates the potential decrease in the between-chains variability \(B\) with respect to the within-chain variability \(W\). If \(\hat{R}\) is large, then longer simulation sequences are expected to either decrease \(B\) or increase \(W\) because the simulations have not yet explored the full posterior distribution. As the article Brooks & Gelman (1998) have suggested, if \(\hat{R} < 1.2\) for all model parameters, one can be fairly confident that convergence has been reached. Otherwise, longer chains or other means for improving the convergence may be needed. Even more reassuring is to apply the more stringent condition \(\hat{R} < 1.1\).
4.2.2 Compute \(\hat{R}\)
We evaluate the convergence of the MCMC output in which four parallel chains are run (with starting values that are over dispersed relative to the posterior distribution). Convergence is diagnosed when the four chains have ‘forgotten’ their initial values, and the output from all chains is indistinguishable. If the convergence diagnostic gives values of potential scale reduction factor (PSRF) or \(\hat{R}\) substantially above 1, its indicates lack of convergence.
# Number of latent variables
n_latent <- mod_Fungi[[1]]$model_spec$n_latent
# Results from list to mcmc.list format
burnin <- mod_Fungi[[1]]$model_spec$burnin
ngibbs <- burnin + mod_Fungi[[1]]$model_spec$mcmc
thin <- mod_Fungi[[1]]$model_spec$thin
require(coda)
arr2mcmc <- function(x) {
return(mcmc(as.data.frame(x),
start=burnin+1 , end=ngibbs, thin=thin))
}
mcmc_list_alpha <- mcmc.list(lapply(lapply(mod_Fungi,"[[","mcmc.alpha"), arr2mcmc))
mcmc_list_V_alpha <- mcmc.list(lapply(lapply(mod_Fungi,"[[","mcmc.V_alpha"), arr2mcmc))
mcmc_list_V_alpha <- mcmc.list(lapply(lapply(mod_Fungi,"[[","mcmc.V_alpha"), arr2mcmc))
mcmc_list_param <- mcmc.list(lapply(lapply(mod_Fungi,"[[","mcmc.sp"), arr2mcmc))
mcmc_list_lv <- mcmc.list(lapply(lapply(mod_Fungi,"[[","mcmc.latent"), arr2mcmc))
mcmc_list_beta <- mcmc_list_param[,grep("beta",colnames(mcmc_list_param[[1]]))]
mcmc_list_beta0 <- mcmc_list_beta[,grep("Intercept", colnames(mcmc_list_beta[[1]]))]
mcmc_list_lambda <- mcmc.list(
lapply(mcmc_list_param[, grep("lambda",
colnames(mcmc_list_param[[1]]),
value=TRUE)], arr2mcmc))
mcmc_list_Deviance <- mcmc.list(lapply(lapply(mod_Fungi,"[[","mcmc.Deviance"), arr2mcmc))
# Rhat
psrf_alpha <- mean(gelman.diag(mcmc_list_alpha, multivariate=FALSE)$psrf[,2])
psrf_V_alpha <- gelman.diag(mcmc_list_V_alpha)$psrf[,2]
psrf_beta0 <- mean(gelman.diag(mcmc_list_beta0)$psrf[,2])
psrf_beta <- mean(gelman.diag(mcmc_list_beta[,grep("Intercept", colnames(mcmc_list_beta[[1]]), invert=TRUE)])$psrf[,2])
psrf_lambda_diag <- mean(gelman.diag(mcmc_list_lambda[,c(1, n_latent+2)],
multivariate=FALSE)$psrf[,2])
# mean(psrf, na.rm=TRUE) because zeros on upper triangular Lambda matrix give NaN in psrf
psrf_lambda_others <- mean(gelman.diag(mcmc_list_lambda[,-c(1, n_latent+2)],
multivariate=FALSE)$psrf[,2],
na.rm=TRUE)
psrf_lv <- mean(gelman.diag(mcmc_list_lv, multivariate=FALSE)$psrf[,2])
Rhat <- data.frame(Rhat=c(psrf_alpha, psrf_V_alpha, psrf_beta0, psrf_beta,
psrf_lambda_diag, psrf_lambda_others, psrf_lv),
Variable=c("alpha", "Valpha", "beta0", "beta",
"lambda \n on the diagonal", "others lambda", "W"))
# Barplot
library(ggplot2)
ggplot(Rhat, aes(x=Variable, y=Rhat)) +
ggtitle("Averages of Rhat obtained for each type of parameter") +
theme(plot.title = element_text(hjust = 0.5, size=15)) +
theme(plot.margin = margin(t = 0.1, r = 0.2, b = 0.1, l = 0.1,"cm")) +
geom_bar(fill="skyblue", stat = "identity") +
geom_text(aes(label=round(Rhat,2)), vjust=0, hjust=-0.1) +
ylim(0, max(Rhat$Rhat)+0.1) +
geom_hline(yintercept=1, color='red') +
coord_flip() 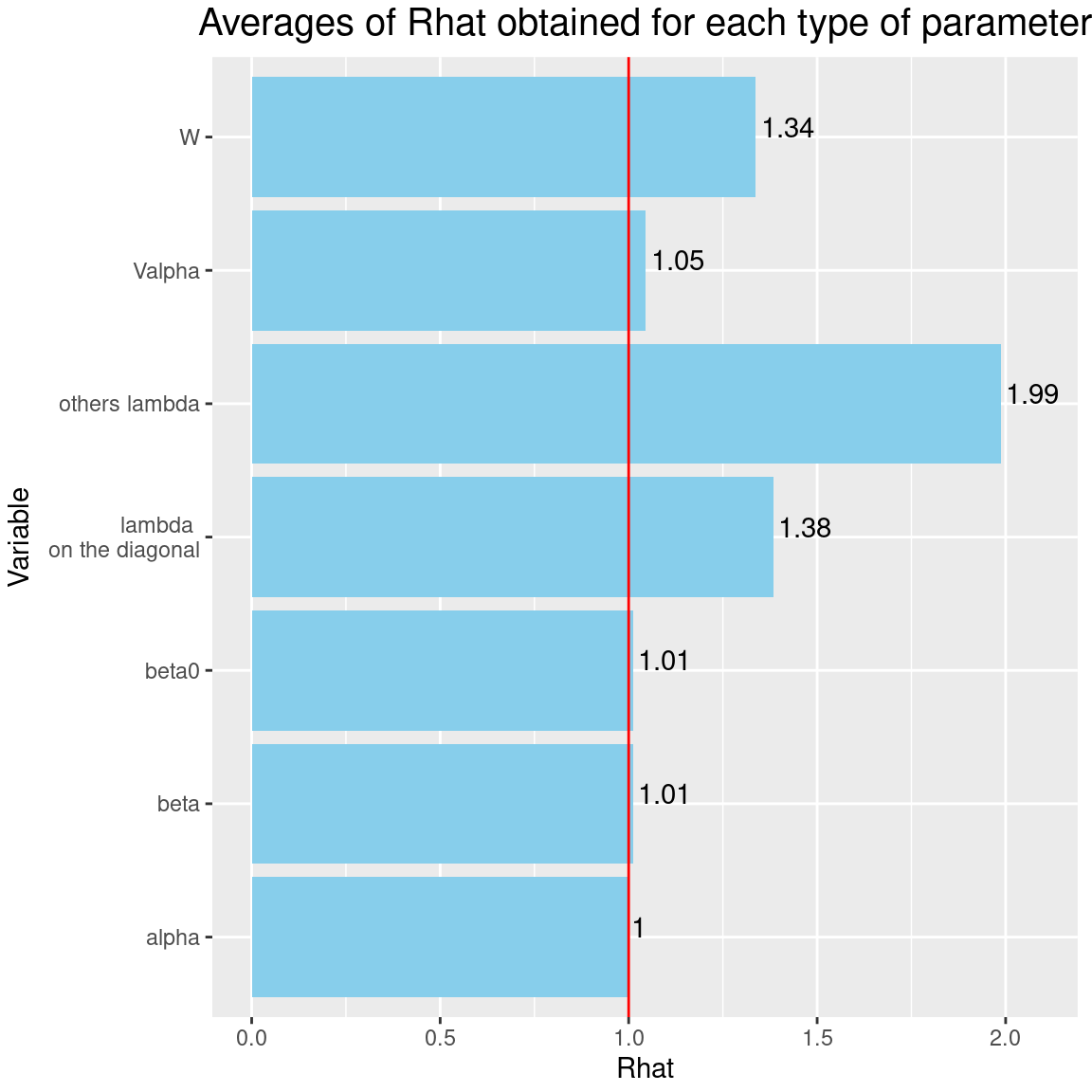
It can be seen that the \(\hat{R}\)s associated with the factor loadings \(\lambda\) are well above 1, which indicates a lack of convergence for these parameters, even after \(15000\) iterations including \(5000\) burn-in.
4.3 Analysis of the results
4.3.1 Representation of results
We visually evaluate the convergence of MCMCs by representing the trace and density a posteriori of some estimated parameters.
np <- nrow(mod_Fungi[[1]]$model_spec$beta_start)
nchains <- length(mod_Fungi)
## beta_j of the first two species
par(mfrow=c(2,2))
plot(mcmc_list_beta[,1:(2*np)],
auto.layout=FALSE)
## lambda_j of the first two species
n_latent <- mod_Fungi[[1]]$model_spec$n_latent
par(mfrow=c(2,2))
plot(mcmc_list_lambda[,1:(2*n_latent)],
auto.layout=FALSE)
## Latent variables W_i for the first two sites
par(mfrow=c(2,2))
nsite <- ncol(mod_Fungi[[1]]$mcmc.alpha)
plot(mcmc_list_lv[, c(1:2, 1:2 + nsite)],
auto.layout=FALSE)
## alpha_i of the first two sites
plot(mcmc_list_alpha[,1:2])
## V_alpha
par(mfrow=c(2,2))
plot(mcmc_list_V_alpha,
auto.layout=FALSE)
## Deviance
plot(mcmc_list_Deviance,
auto.layout=FALSE)
## probit_theta
par(mfrow=c(1,2))
hist(mod_Fungi[[1]]$probit_theta_latent,
col=1,
main = "Predicted probit theta",
xlab ="predicted probit theta")
for(i in 2:nchains){
hist(mod_Fungi[[i]]$probit_theta_latent,
add=TRUE, col=i)
}
# occurrence probabilities theta
hist(mod_Fungi[[1]]$theta_latent,
col=1, main = "Predicted theta",
xlab ="predicted theta")
for(i in 2:nchains){
hist(mod_Fungi[[i]]$theta_latent,
add=TRUE, col=i)
}
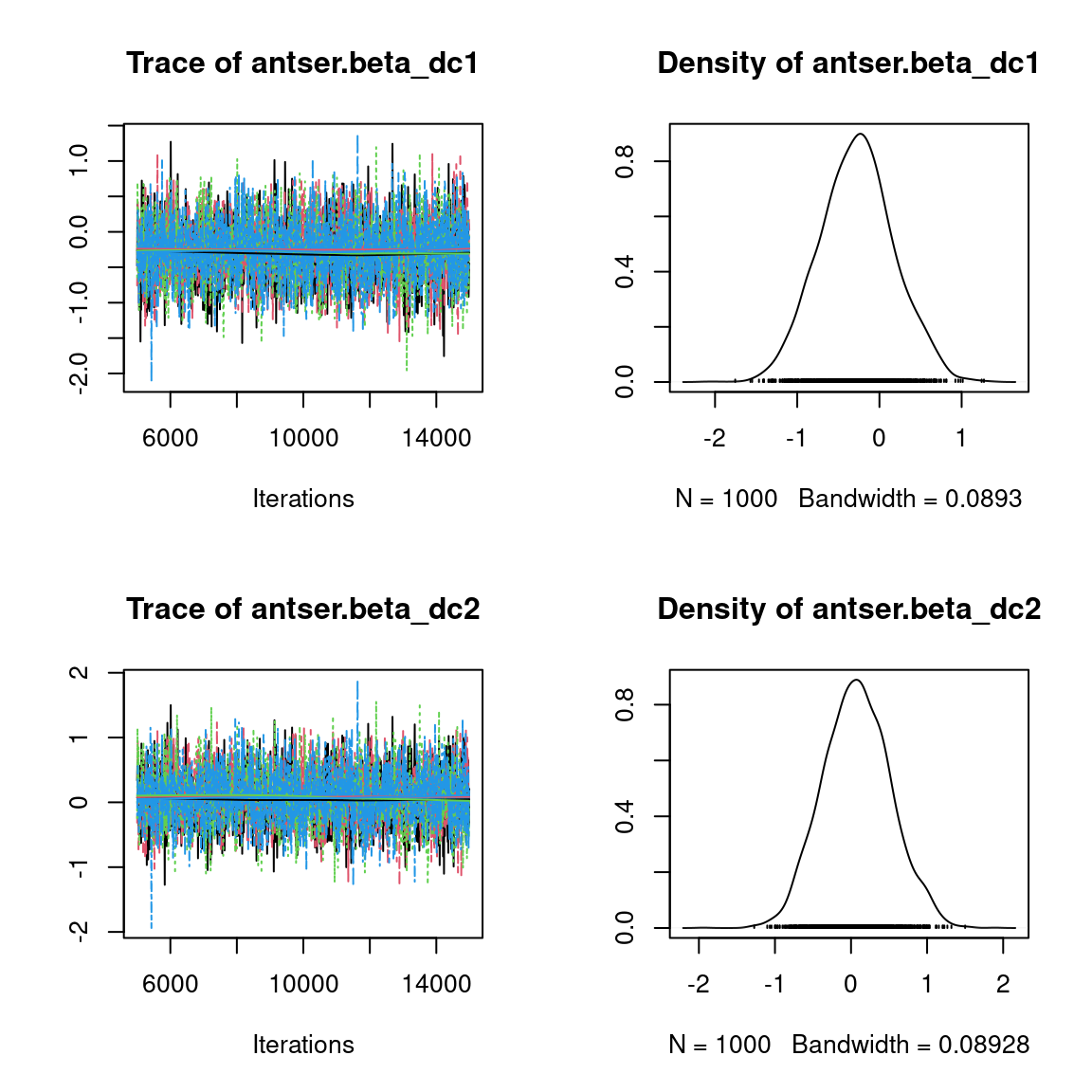
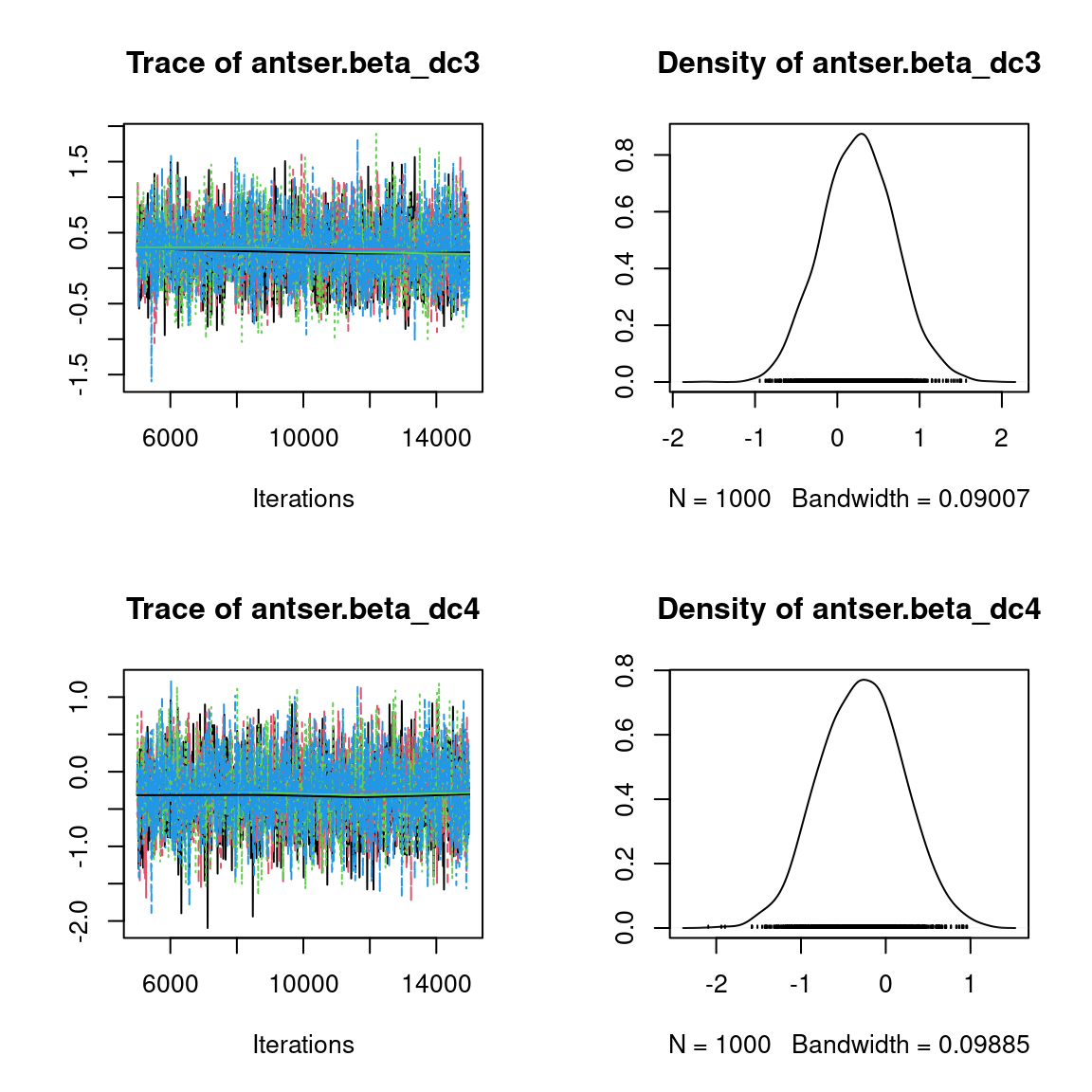
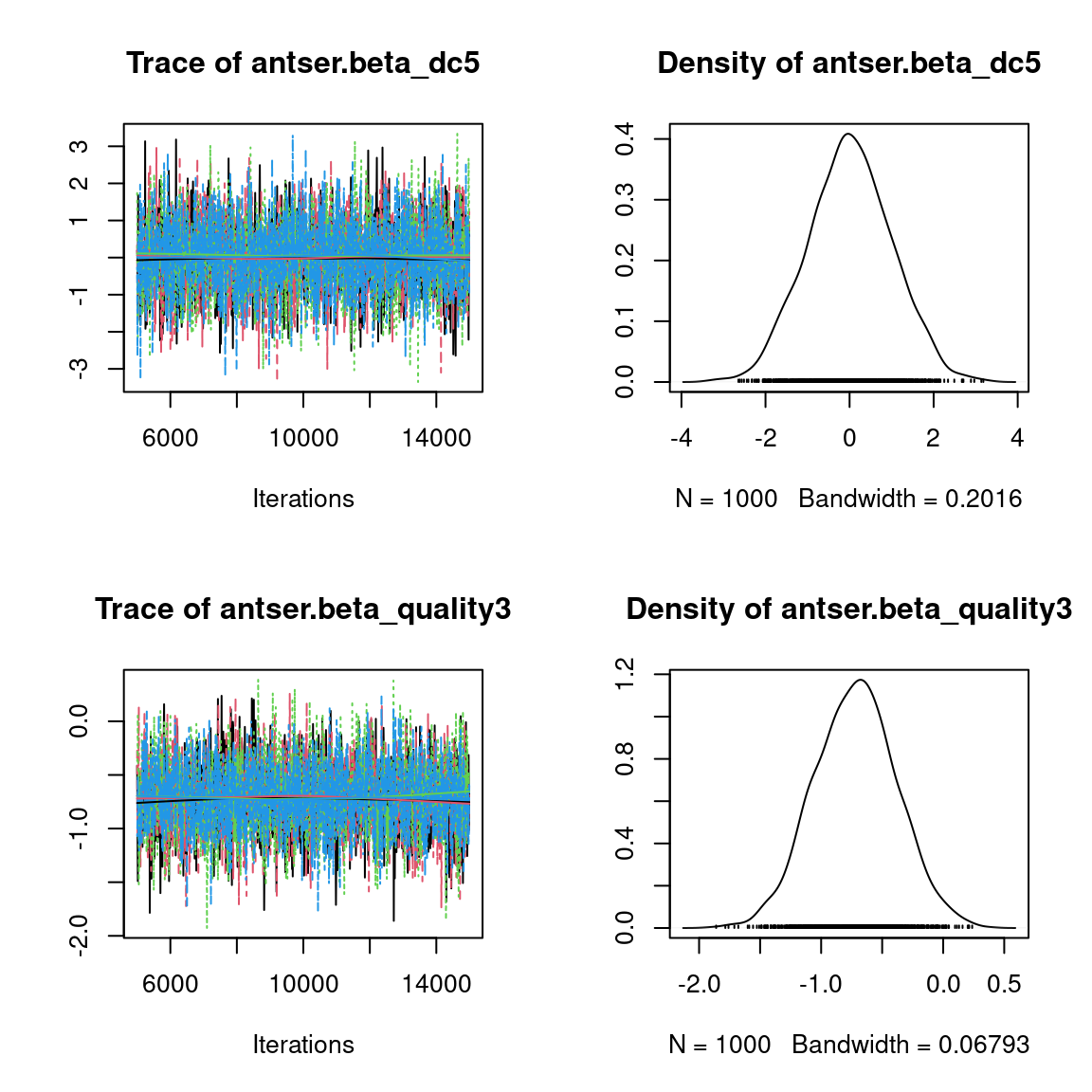
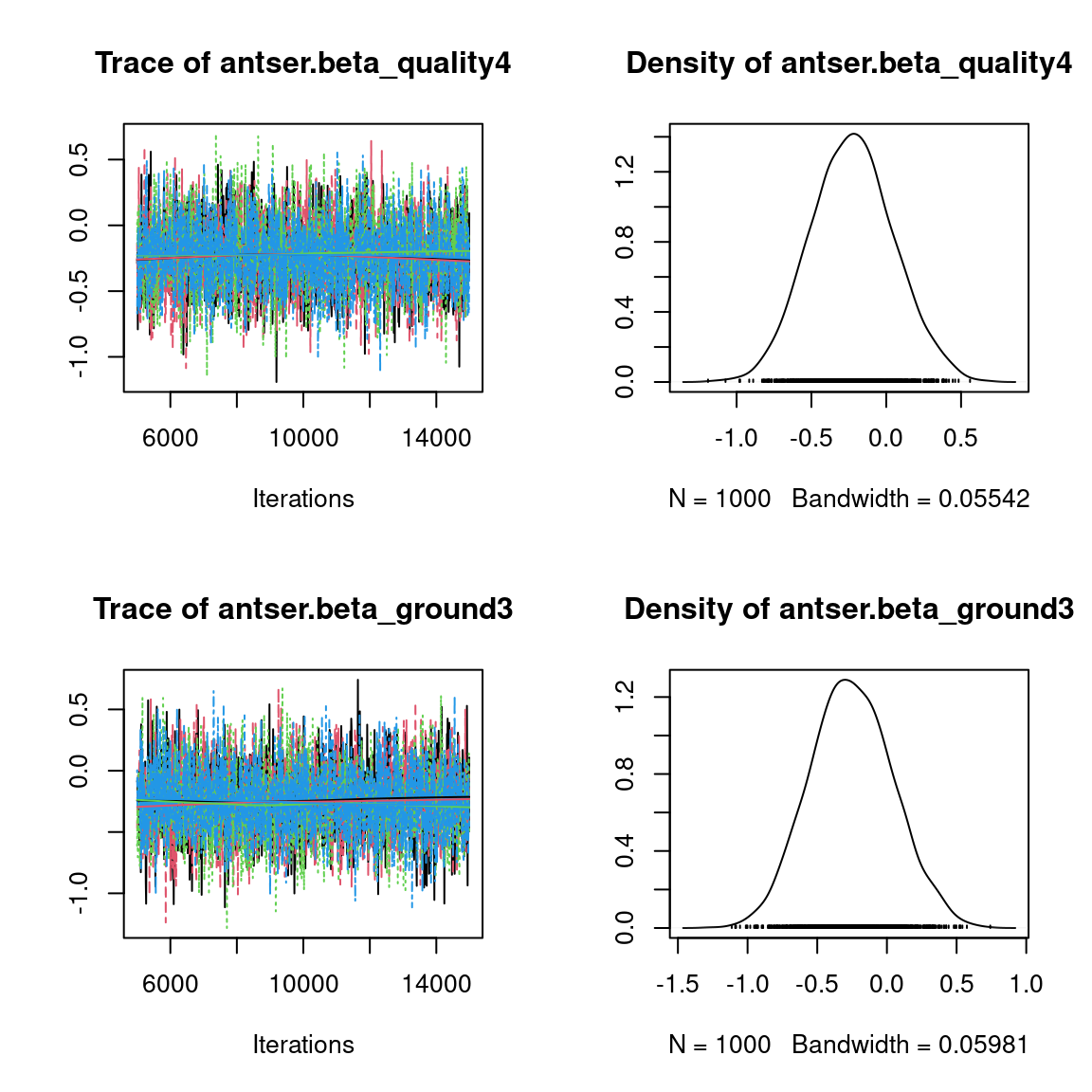
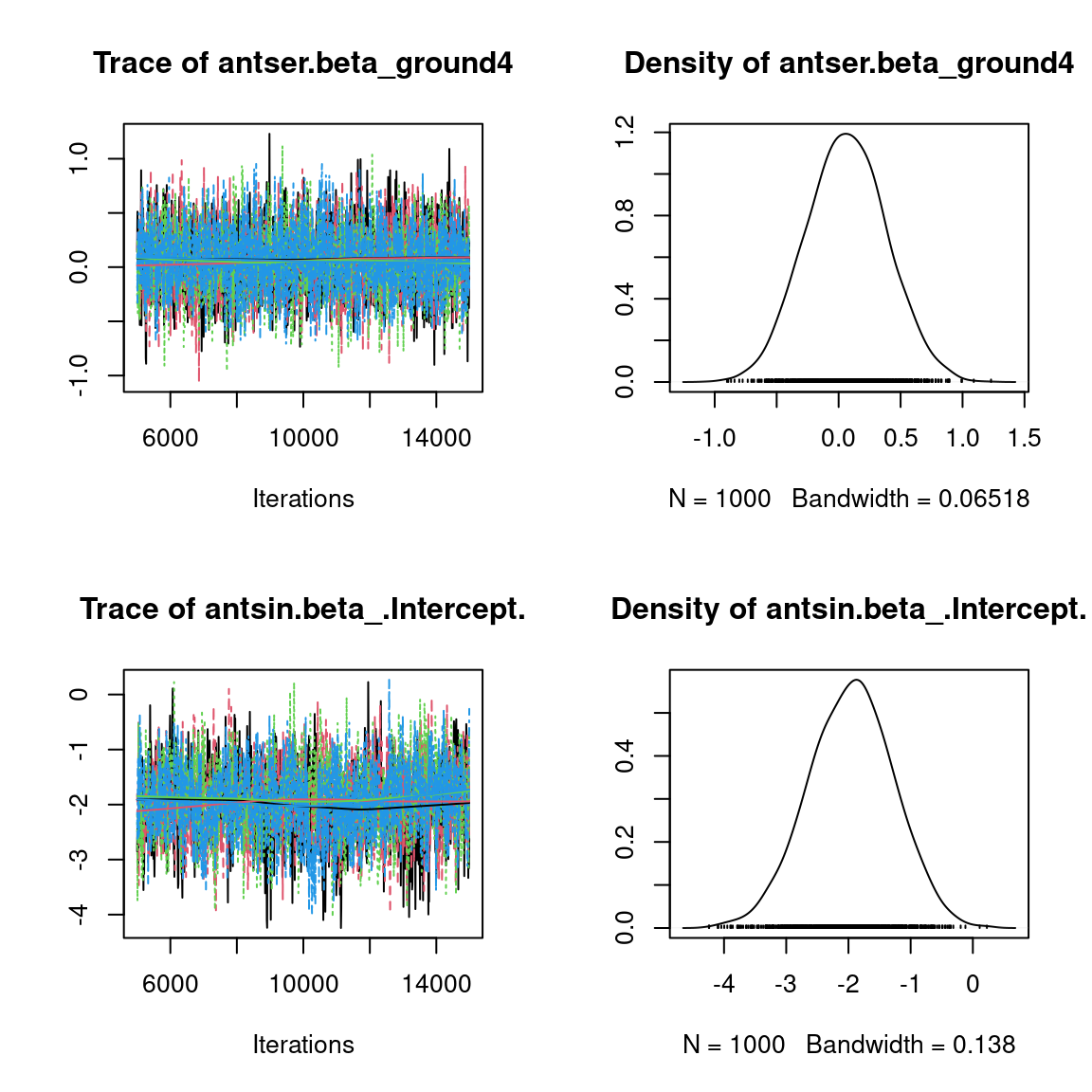
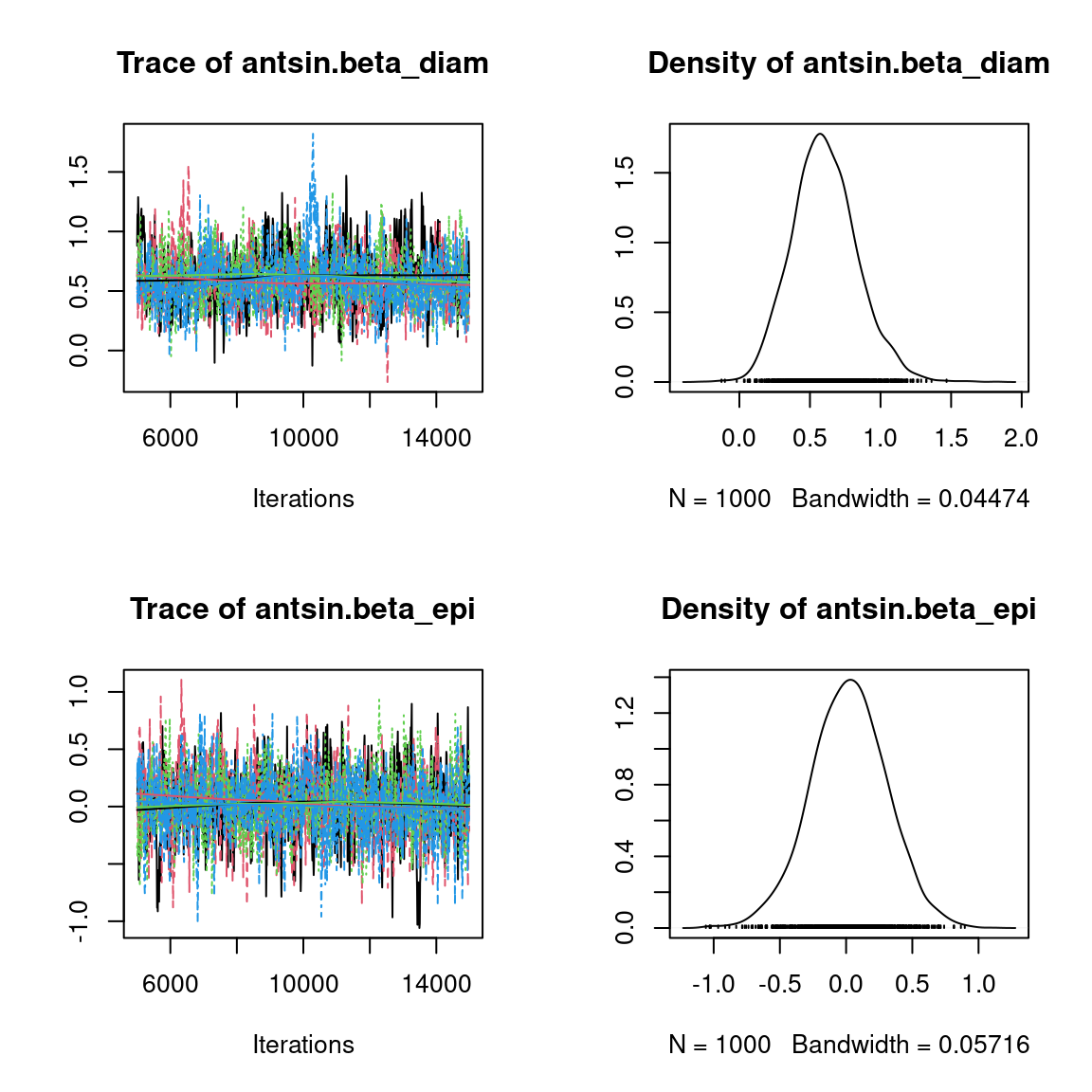
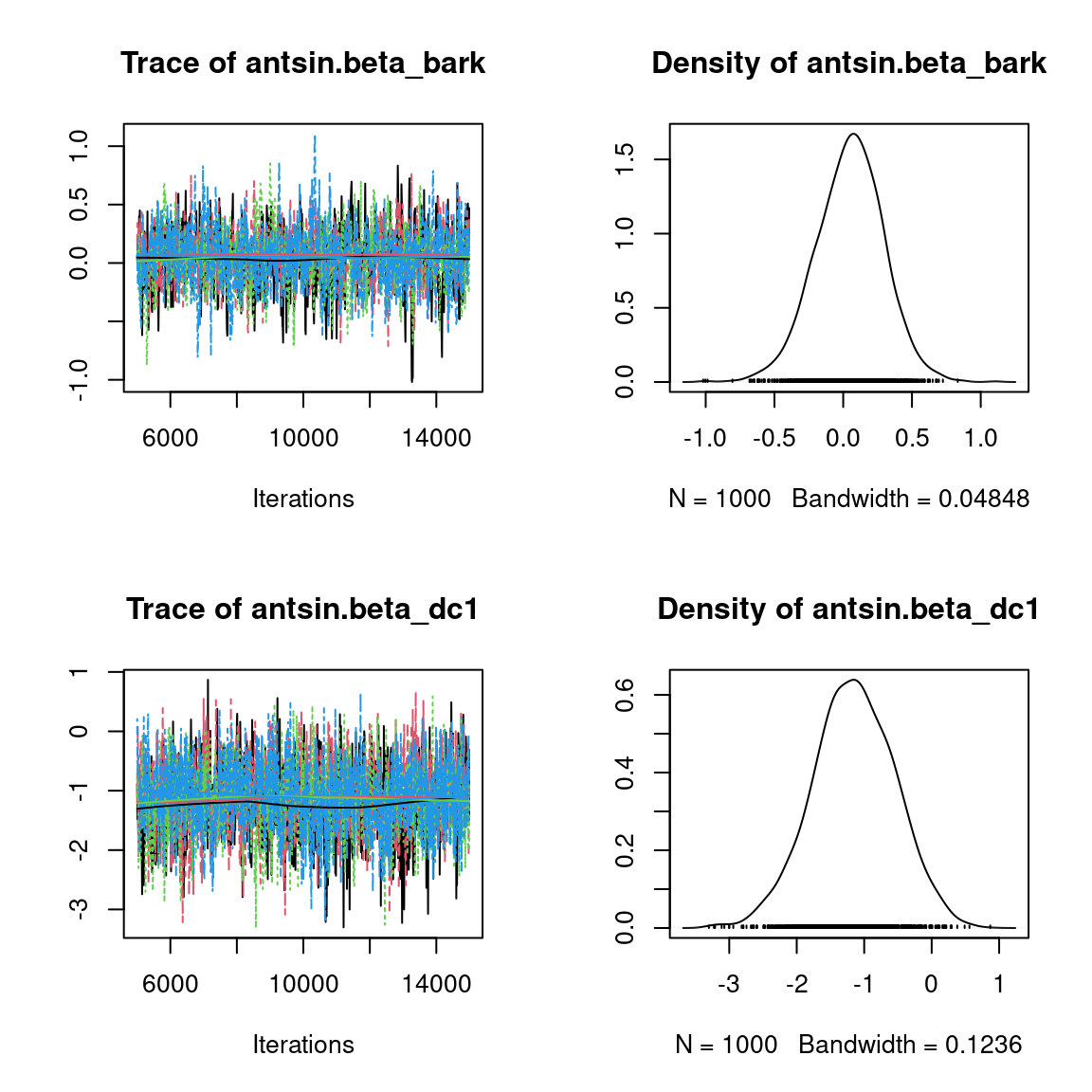
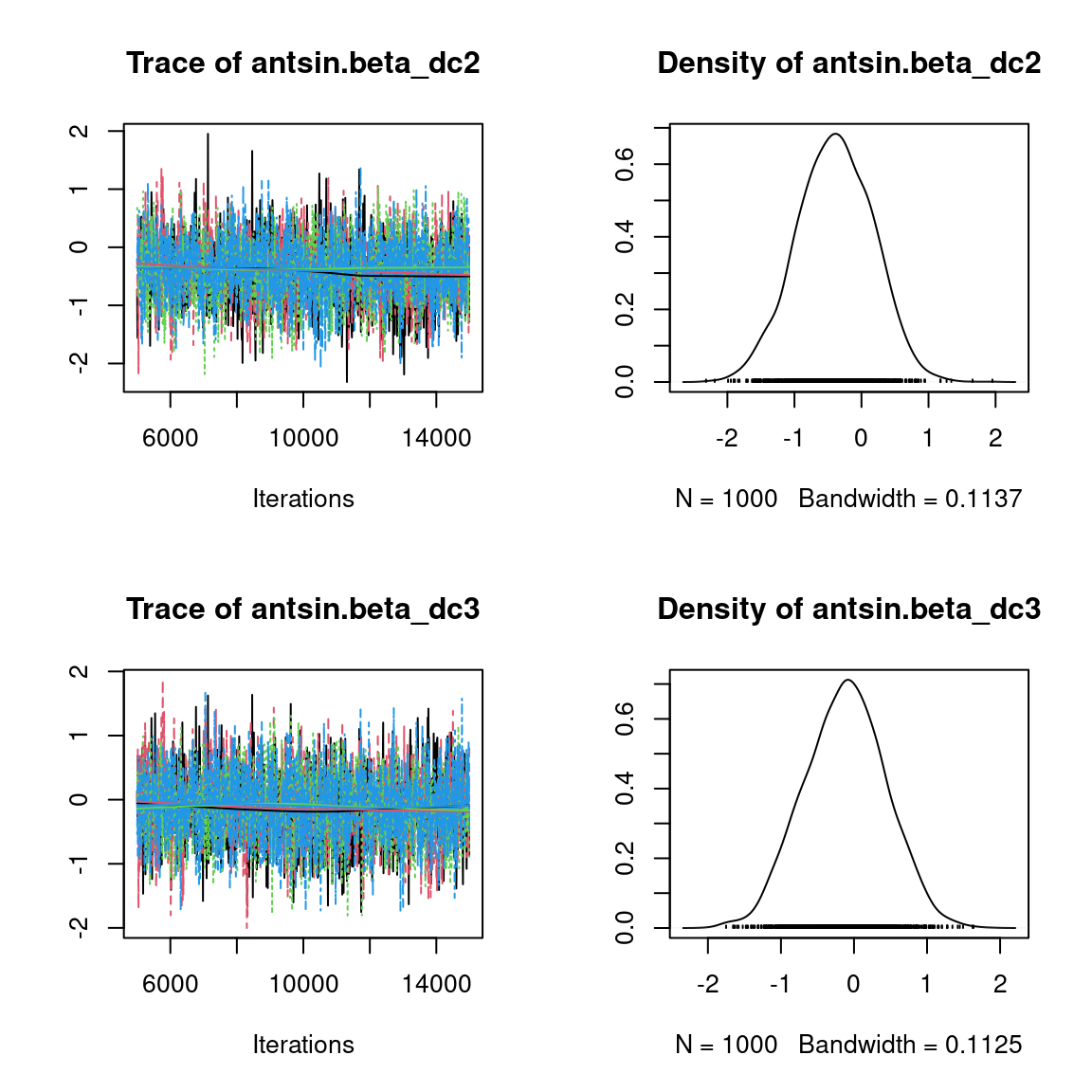
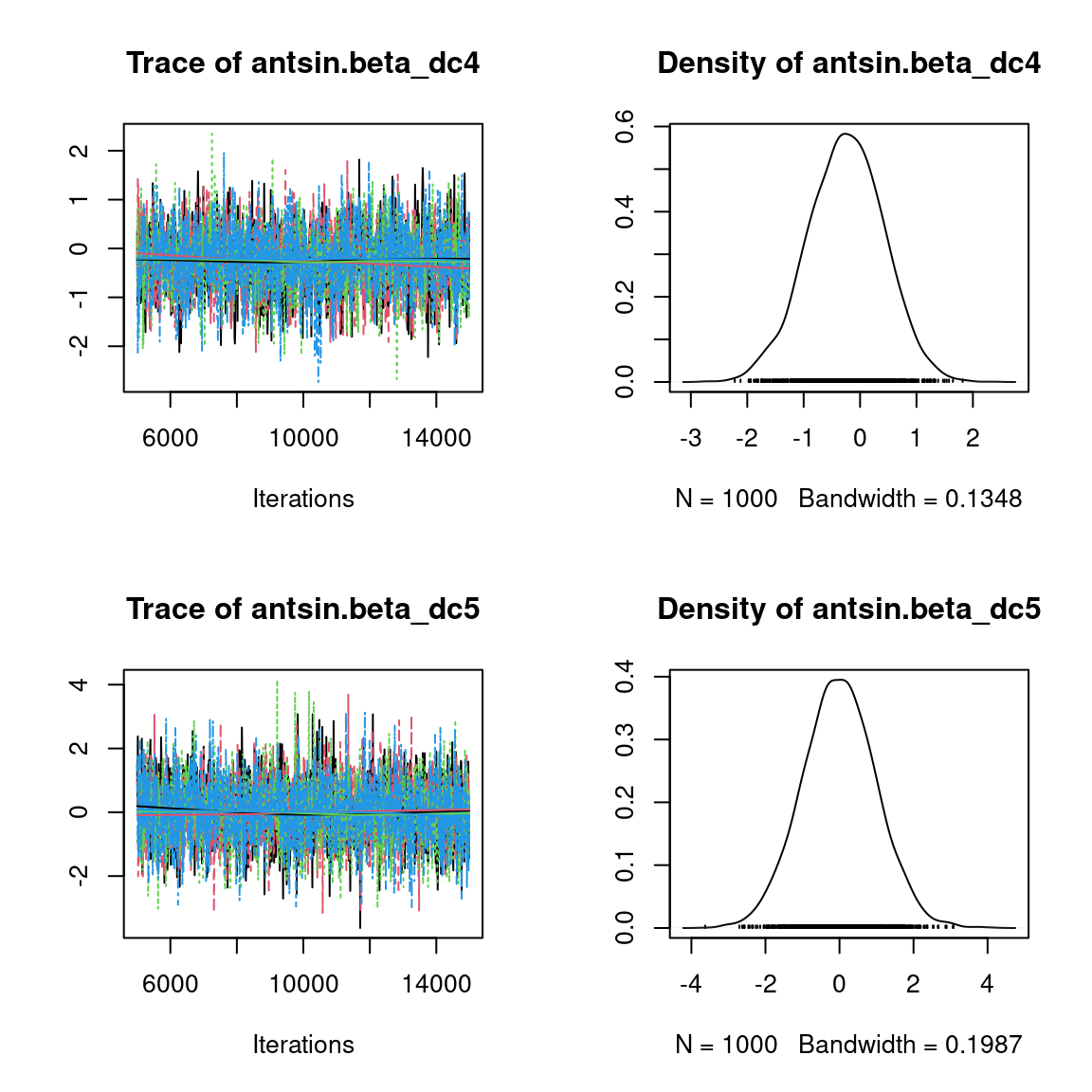
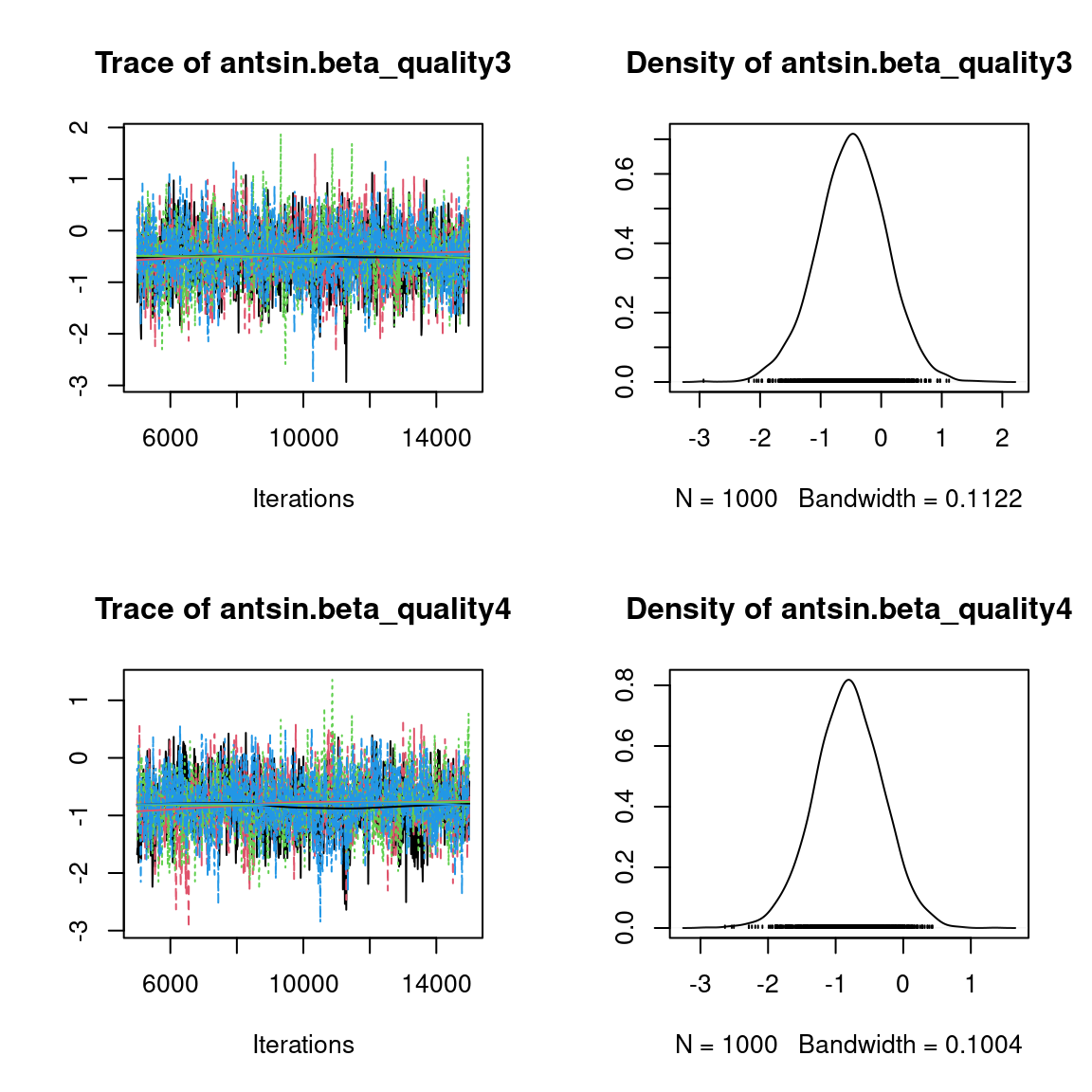
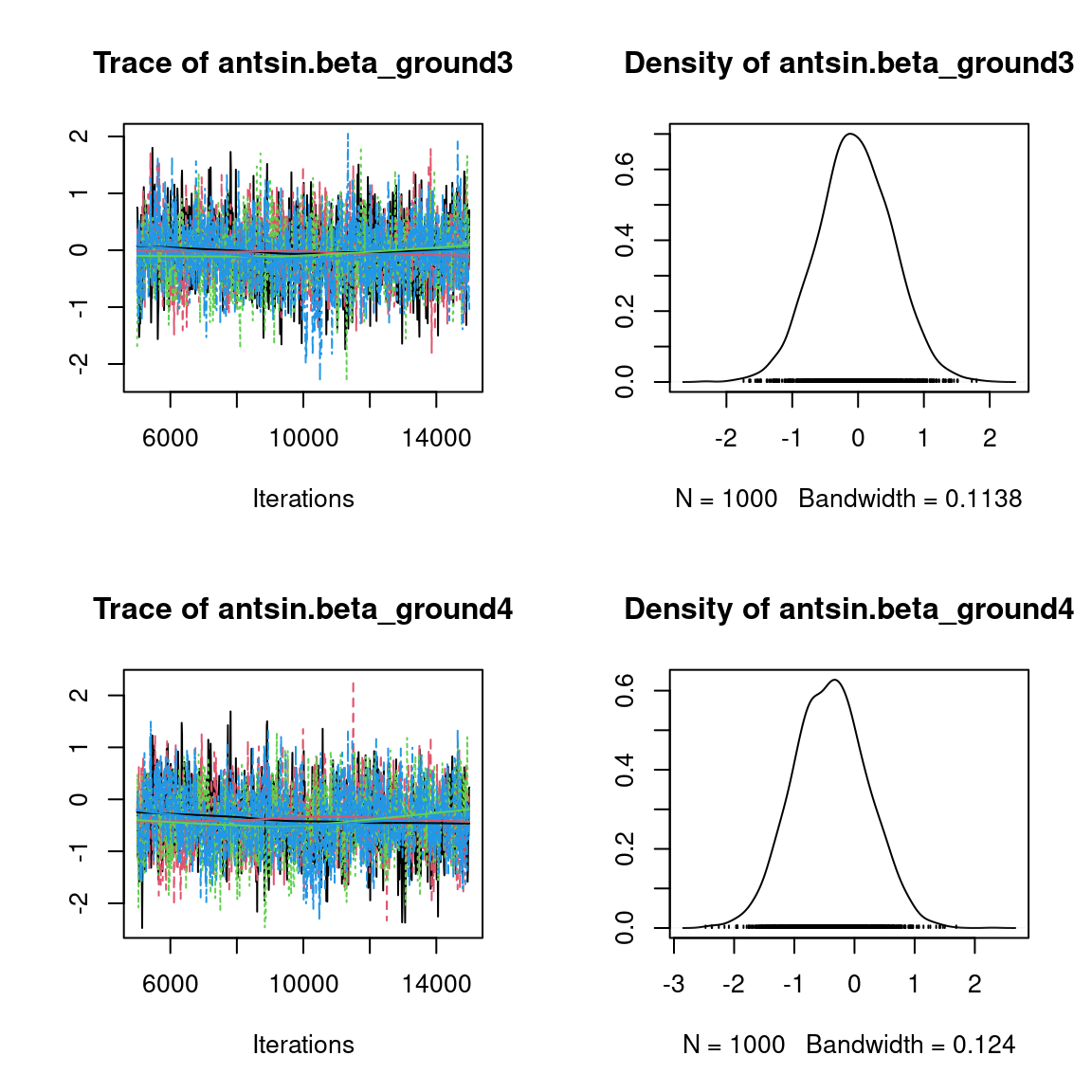
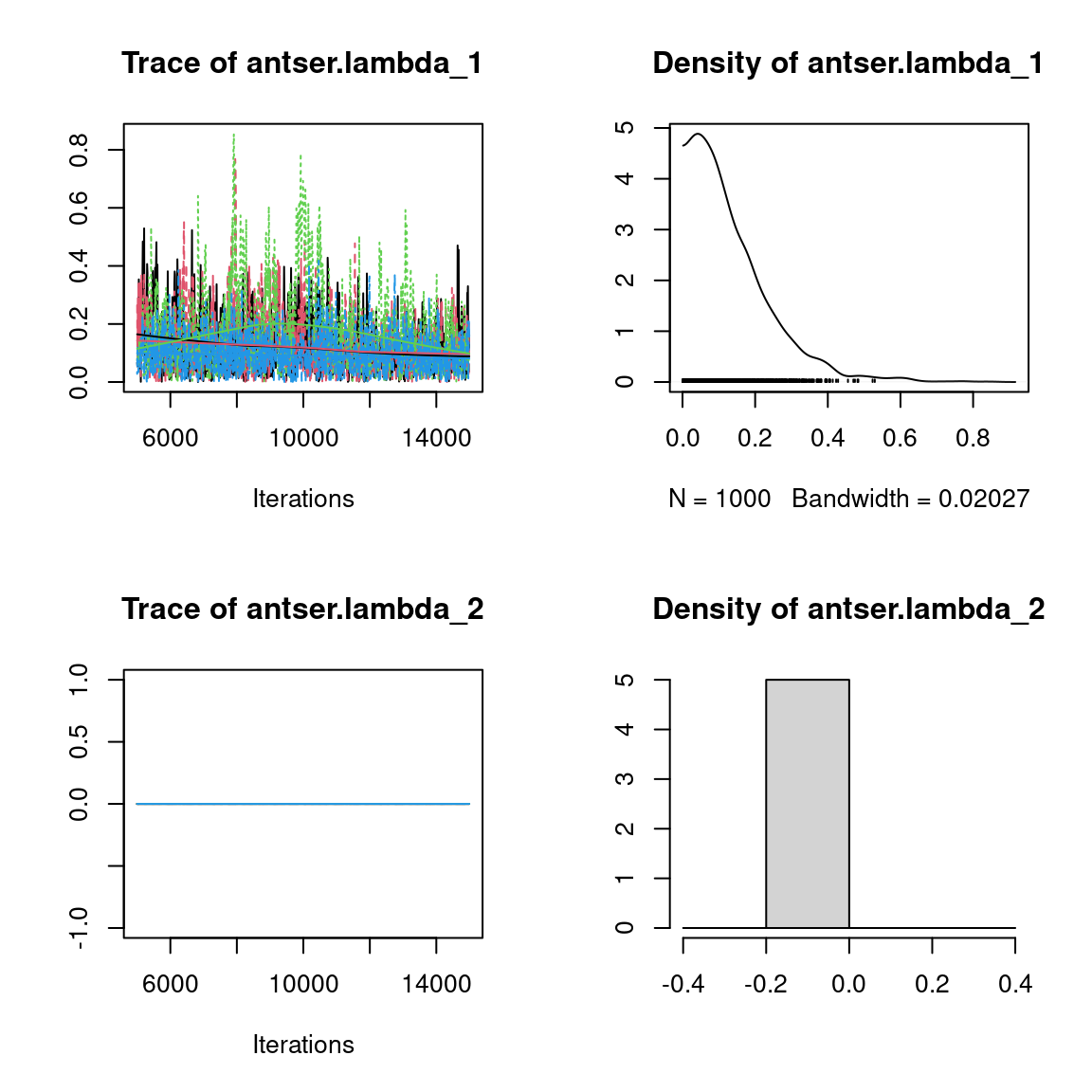
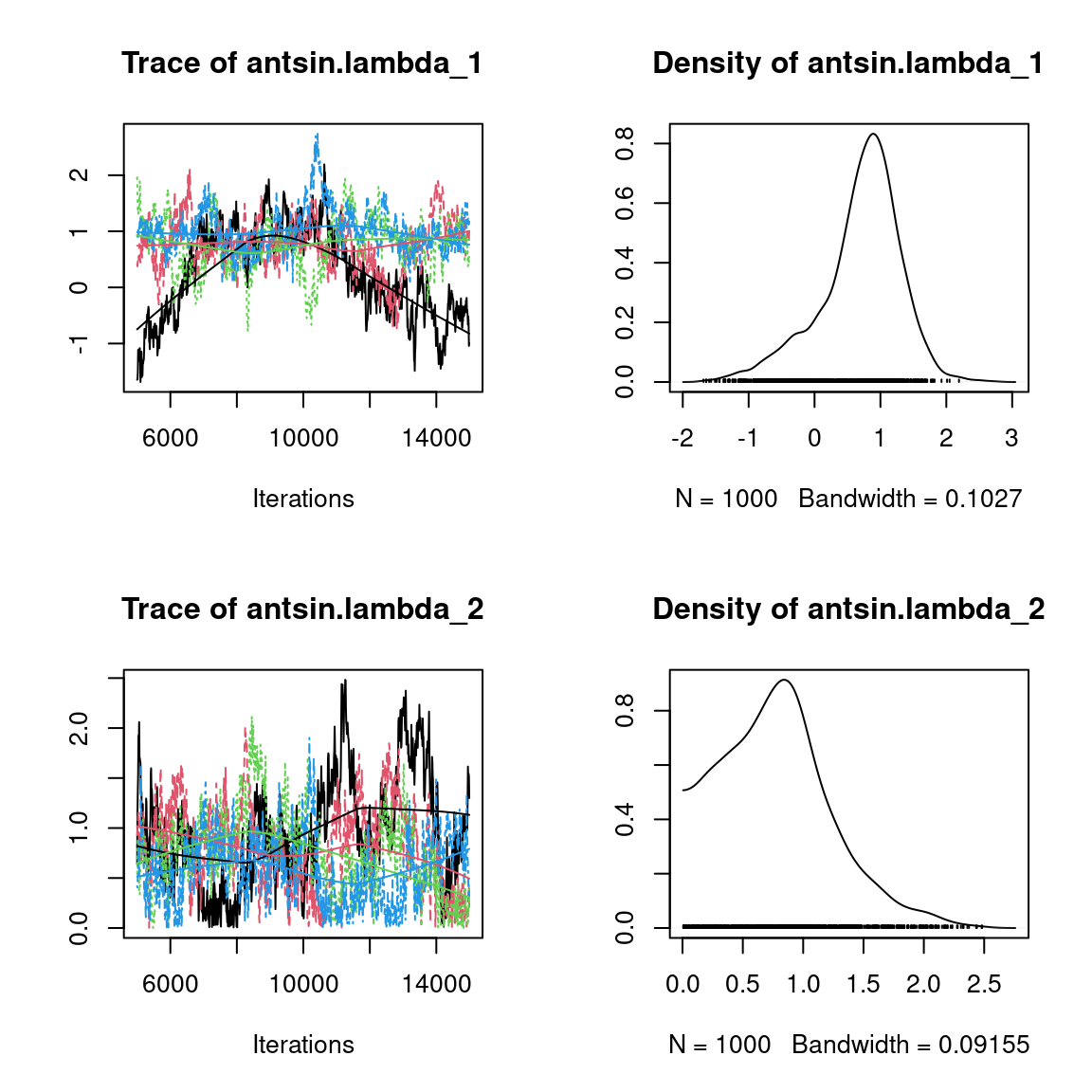
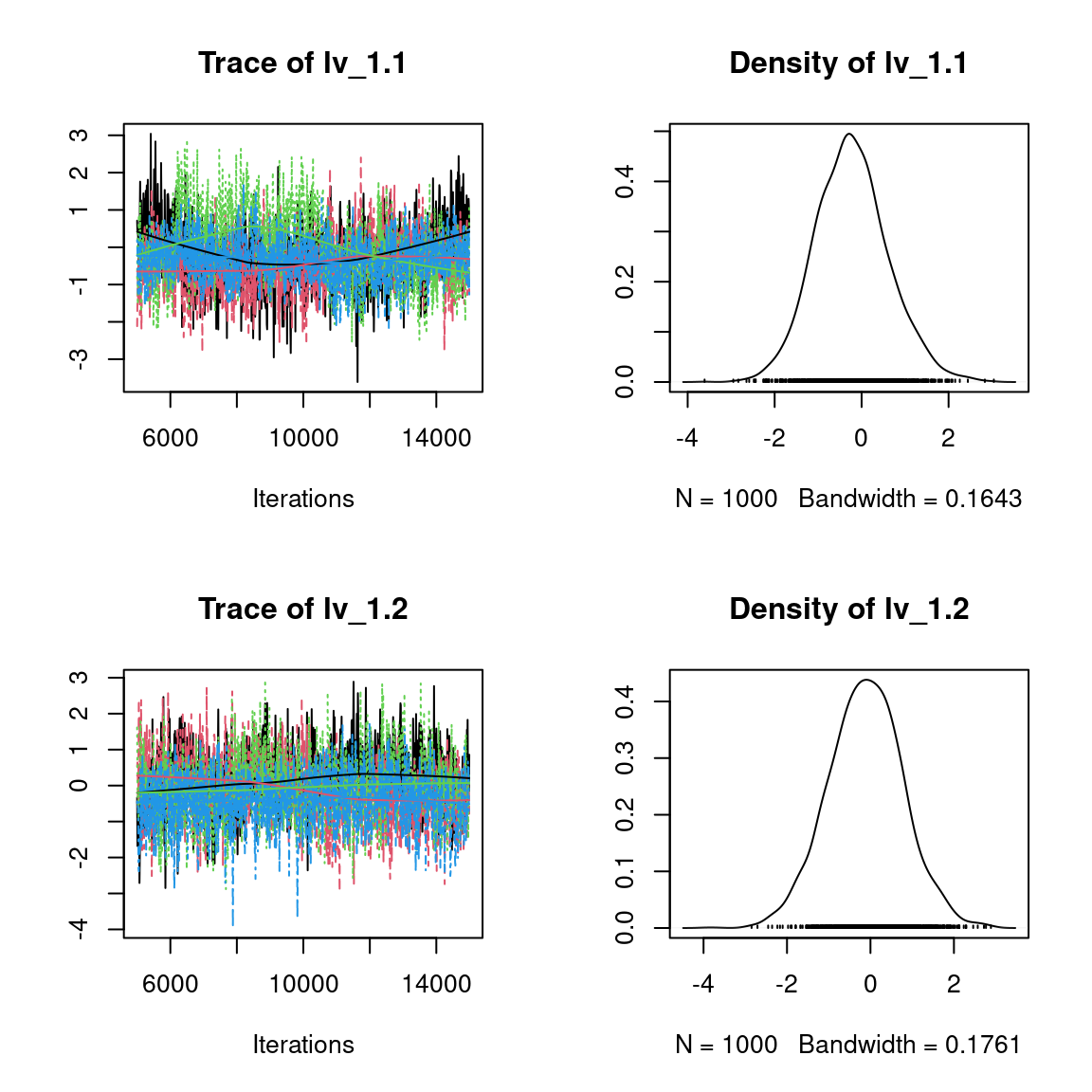
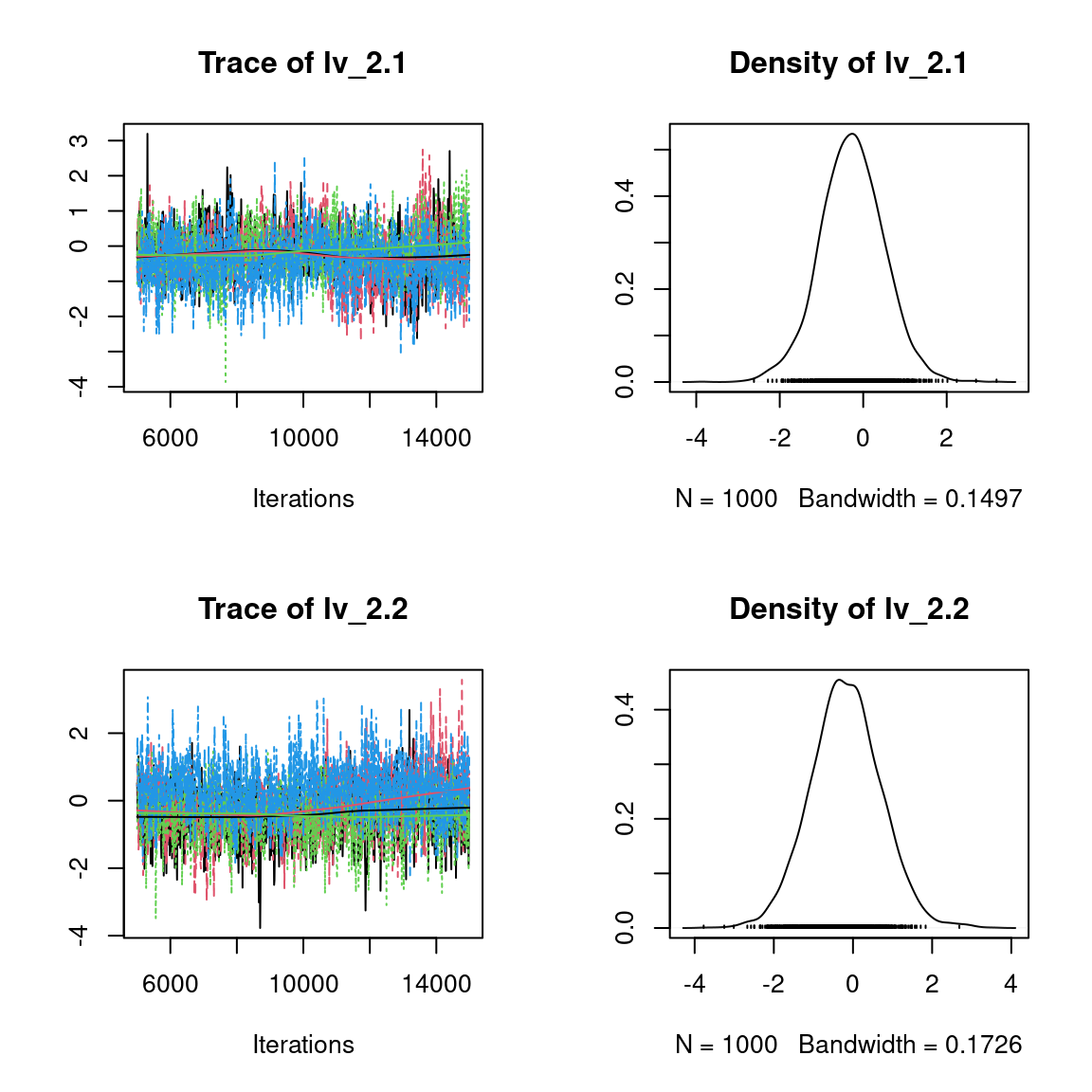
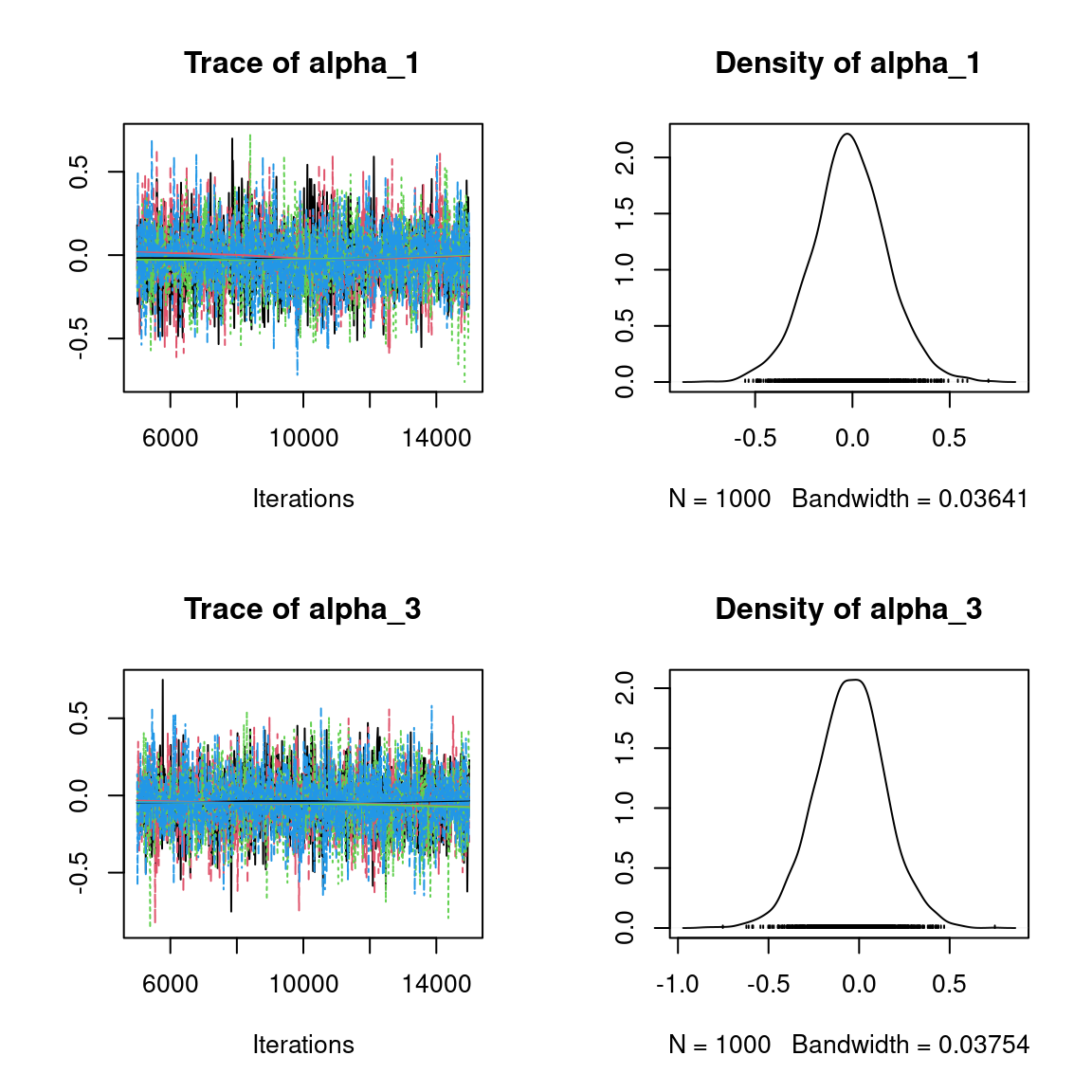
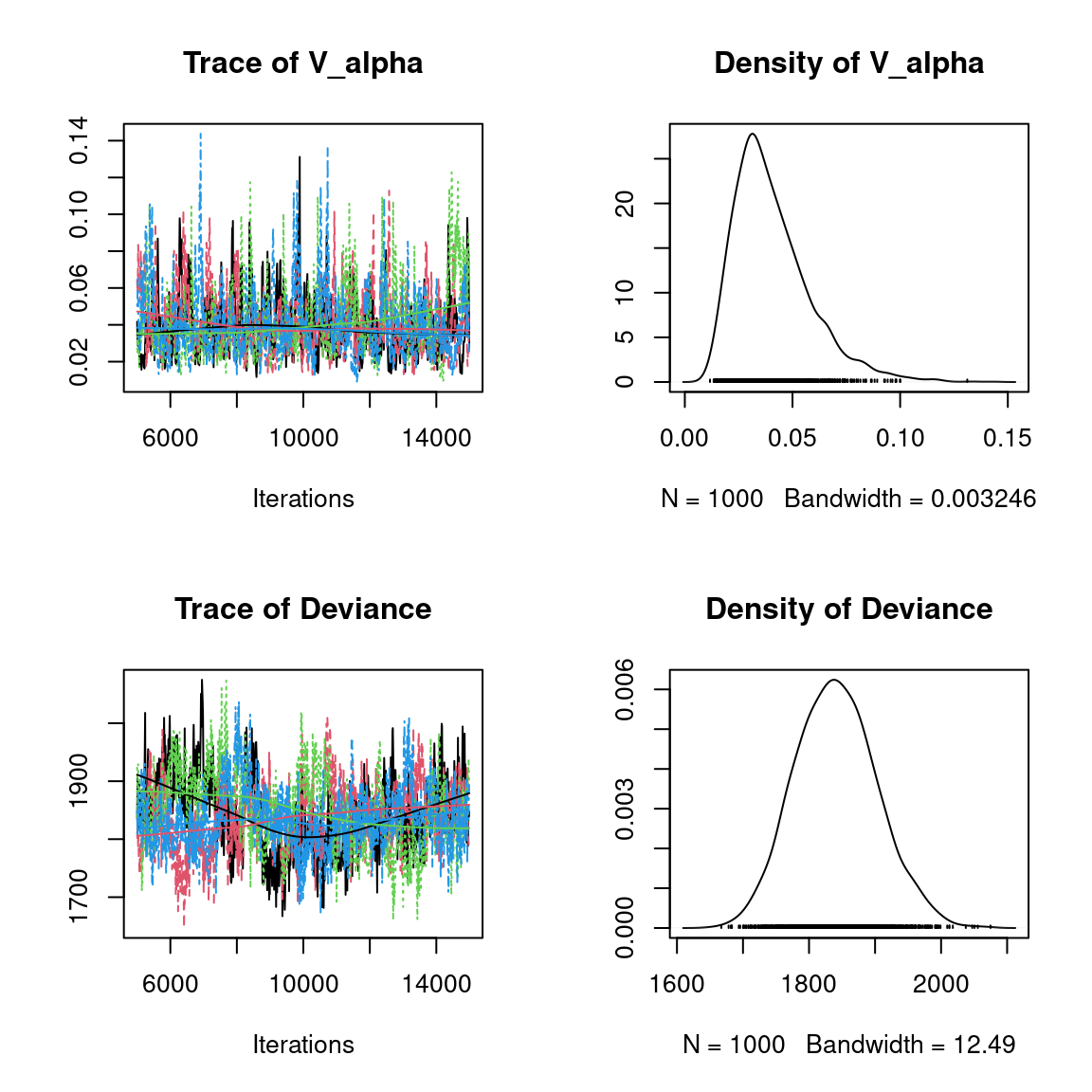
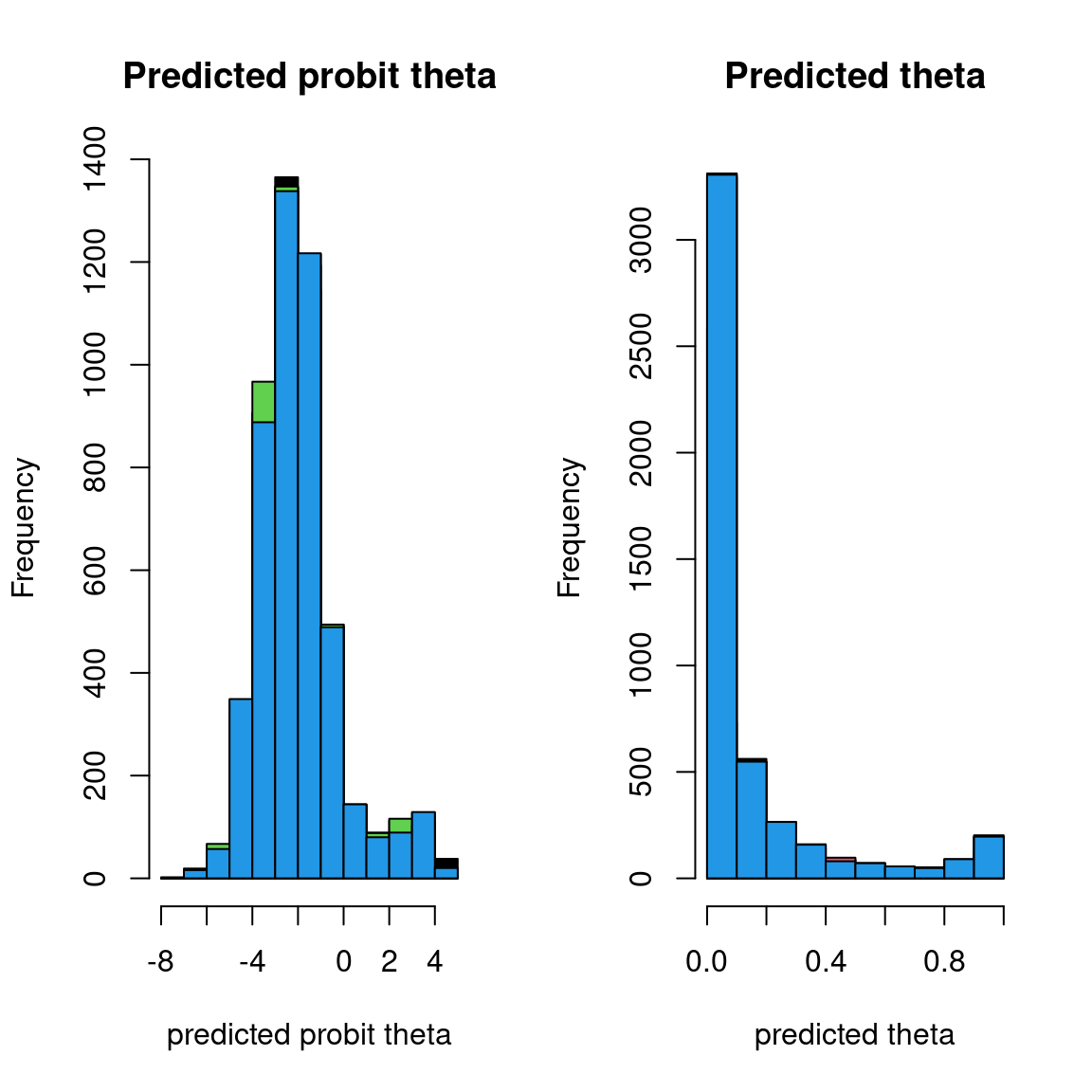
Overall, the traces and the densities of the parameters indicate the convergence of the algorithm. Indeed, we observe on the traces that the values oscillate around averages without showing an upward or downward trend and we see that the densities are quite smooth and for the most part of Gaussian form, except for the factor loadings \(\lambda\).
In fact the values estimated for \(\lambda\) differ between the four chains and within each chain. The traces of these parameters oscillate very strongly showing upward or downward trends and we see that the densities are not of Gaussian form, as expected.
This lack of convergence can be explained by the arbitrary choice of the species constrained to have positive values of \(\lambda\) as being the first ones in the presence-absence data set. Indeed, the constrained species are supposed to be the ones that structure most clearly each latent axis but by arbitrarily choosing them this is not necessarily the case.
4.3.2 Matrice of residual correlations
After fitting the jSDM with latent variables, the full species residual correlation matrix \(R=(R_{ij})^{i=1,\ldots, nspecies}_{j=1,\ldots, nspecies}\) can be derived from the covariance in the latent variables such as : \[\Sigma_{ij} = \lambda_i^T .\lambda_j\], then we compute correlations from covariances : \[R_{i,j} = \frac{\Sigma_{ij}}{\sqrt{\Sigma _{ii}\Sigma _{jj}}}\].
We use the get_residual_cor() function to compute the residual correlation matrix \(R\) for each chain and we display the average of the \(R\) matrices for all chains:
nsp <- ncol(mod_Fungi[[1]]$model_spec$beta_start)
# Average residual correlation matrix on the four mcmc chains
R <- matrix(0, nsp, nsp)
for(i in 1:nchains){
R <- R + get_residual_cor(mod_Fungi[[i]])$cor.mean/nchains
}
colnames(R) <- rownames(R) <- colnames(PA_Fungi)
R.reorder <- corrplot::corrMatOrder(R, order = "FPC", hclust.method = "average")
corrplot::corrplot(R[R.reorder, R.reorder], diag = F, type = "lower",
method = "color", mar = c(1,1,3,1),
tl.srt = 45, tl.cex = 0.7,
title = "Residual Correlation Matrix from LVM")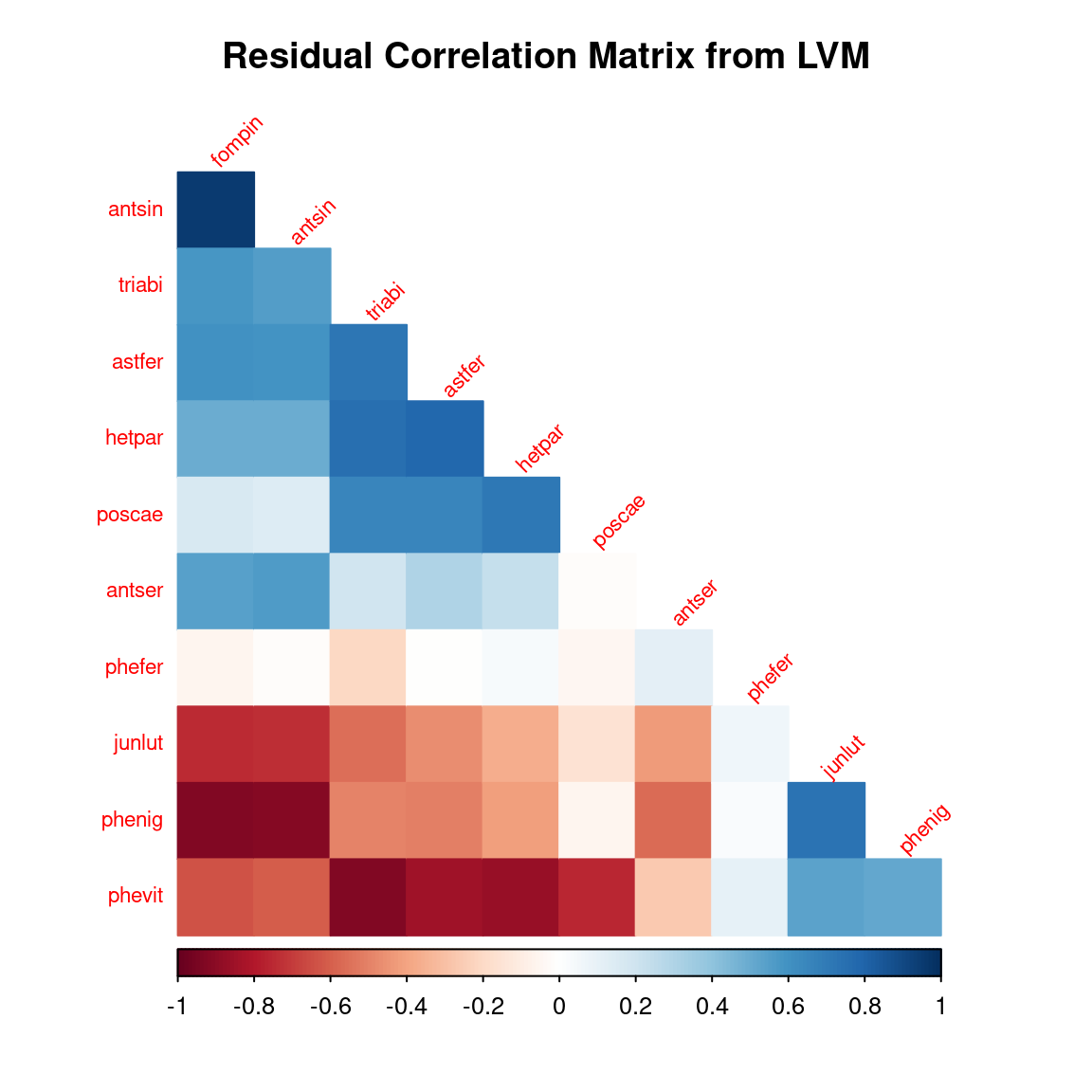
5 Fitting JSDM with the chosen constrained species
5.1 Parameter inference
As a second step we use the jSDM_binomial_probit_sp_constrained() function to fit the JSDM in parallel, to obtain four MCMC chains of parameters.
At first, the function fit a JSDM with the constrained species arbitrarily chosen as the first ones in the presence-absence data-set.
Then, the function evaluates the convergence of MCMC \(\lambda\) chains using the Gelman-Rubin convergence diagnostic (\(\hat{R}\)). It identifies the species (\(\hat{j}_l\)) having the higher \(\hat{R}\) for \(\lambda_{\hat{j}_l}\). These species drive the structure of the latent axis \(l\). The \(\lambda\) corresponding to this species are constrained to be positive and placed on the diagonal of the \(\Lambda\) matrix for fitting a second model.
This usually improves the convergence of the latent variables and factor loadings. The function returns the parameter posterior distributions for this second model.
# Model
# Inferring model parameters
mod_Fungi_ord <- jSDM::jSDM_binomial_probit_sp_constrained(# Chains
ncores=4,
nchains=4,
# Iteration
burnin=5000,
mcmc=10000,
thin=10,
# Response variable
presence_data = PA_Fungi,
# Explanatory variables
site_formula = ~.,
site_data = Env_Fungi,
# Model specification
n_latent=2,
site_effect="random",
# Starting values
alpha_start=0,
beta_start=0,
lambda_start=0,
W_start=0,
V_alpha=1,
# Priors
shape_Valpha=0.1,
rate_Valpha=0.1,
mu_beta=0,
V_beta=1,
mu_lambda=0,
V_lambda=1,
# Other
seed = seed_mcmc[i],
verbose = 0
)5.2 The Gelman–Rubin convergence diagnostic
5.2.1 Compute \(\hat{R}\)
We evaluate the convergence of the MCMC output in which four parallel chains are run (with starting values that are over dispersed relative to the posterior distribution). Convergence is diagnosed when the four chains have ‘forgotten’ their initial values, and the output from all chains is indistinguishable. If the convergence diagnostic gives values of potential scale reduction factor (PSRF) or \(\hat{R}\) substantially above 1, its indicates lack of convergence.
# Results from list to mcmc.list format
burnin <- mod_Fungi_ord[[1]]$model_spec$burnin
ngibbs <- burnin + mod_Fungi_ord[[1]]$model_spec$mcmc
thin <- mod_Fungi_ord[[1]]$model_spec$thin
require(coda)
arr2mcmc <- function(x) {
return(mcmc(as.data.frame(x),
start=burnin+1 , end=ngibbs, thin=thin))
}
mcmc_list_alpha <- mcmc.list(lapply(lapply(mod_Fungi_ord,"[[","mcmc.alpha"), arr2mcmc))
mcmc_list_V_alpha <- mcmc.list(lapply(lapply(mod_Fungi_ord,"[[","mcmc.V_alpha"), arr2mcmc))
mcmc_list_param <- mcmc.list(lapply(lapply(mod_Fungi_ord,"[[","mcmc.sp"), arr2mcmc))
mcmc_list_lv <- mcmc.list(lapply(lapply(mod_Fungi_ord,"[[","mcmc.latent"), arr2mcmc))
mcmc_list_beta <- mcmc_list_param[,grep("beta",colnames(mcmc_list_param[[1]]))]
mcmc_list_beta0 <- mcmc_list_beta[,grep("Intercept", colnames(mcmc_list_beta[[1]]))]
mcmc_list_lambda <- mcmc.list(
lapply(mcmc_list_param[, grep("lambda",
colnames(mcmc_list_param[[1]]),
value=TRUE)], arr2mcmc))
# Rhat
psrf_alpha <- mean(gelman.diag(mcmc_list_alpha, multivariate=FALSE)$psrf[,2])
psrf_V_alpha <- gelman.diag(mcmc_list_V_alpha)$psrf[,2]
psrf_beta0 <- mean(gelman.diag(mcmc_list_beta0)$psrf[,2])
psrf_beta <- mean(gelman.diag(mcmc_list_beta[,grep("Intercept",
colnames(mcmc_list_beta[[1]]),
invert=TRUE)])$psrf[,2])
id_sp_constrained <- NULL
for(l in 1:n_latent){
id_sp_constrained <- c(id_sp_constrained,
grep(mod_Fungi_ord[[1]]$sp_constrained[l],
varnames(mcmc_list_lambda)))
}
psrf_lambda_diag <- mean(gelman.diag(
mcmc_list_lambda[,id_sp_constrained],
multivariate=FALSE)$psrf[,2],
na.rm=TRUE)
# mean(psrf, na.rm=TRUE) because zeros on upper triangular Lambda matrix give NaN in psrf
psrf_lambda_others <- mean(gelman.diag(mcmc_list_lambda[,-id_sp_constrained],
multivariate=FALSE)$psrf[,2])
psrf_lv <- mean(gelman.diag(mcmc_list_lv,
multivariate=FALSE)$psrf[,2])
Rhat <- data.frame(Rhat=c(psrf_alpha, psrf_V_alpha, psrf_beta0, psrf_beta,
psrf_lambda_diag, psrf_lambda_others, psrf_lv),
Variable=c("alpha", "Valpha", "beta0", "beta",
"lambda \n on the diagonal", "others lambda", "W"))
# Barplot
library(ggplot2)
ggplot2::ggplot(Rhat, aes(x=Variable, y=Rhat)) +
ggtitle("Averages of Rhat obtained for each type of parameter") +
theme(plot.title = element_text(hjust = 0.5, size=15)) +
theme(plot.margin = margin(t = 0.1, r = 0.2, b = 0.1, l = 0.1,"cm")) +
geom_bar(fill="skyblue", stat = "identity") +
geom_text(aes(label=round(Rhat,2)), vjust=0, hjust=-0.1) +
ylim(0, max(Rhat$Rhat)+0.1) +
geom_hline(yintercept=1, color='red') +
coord_flip() 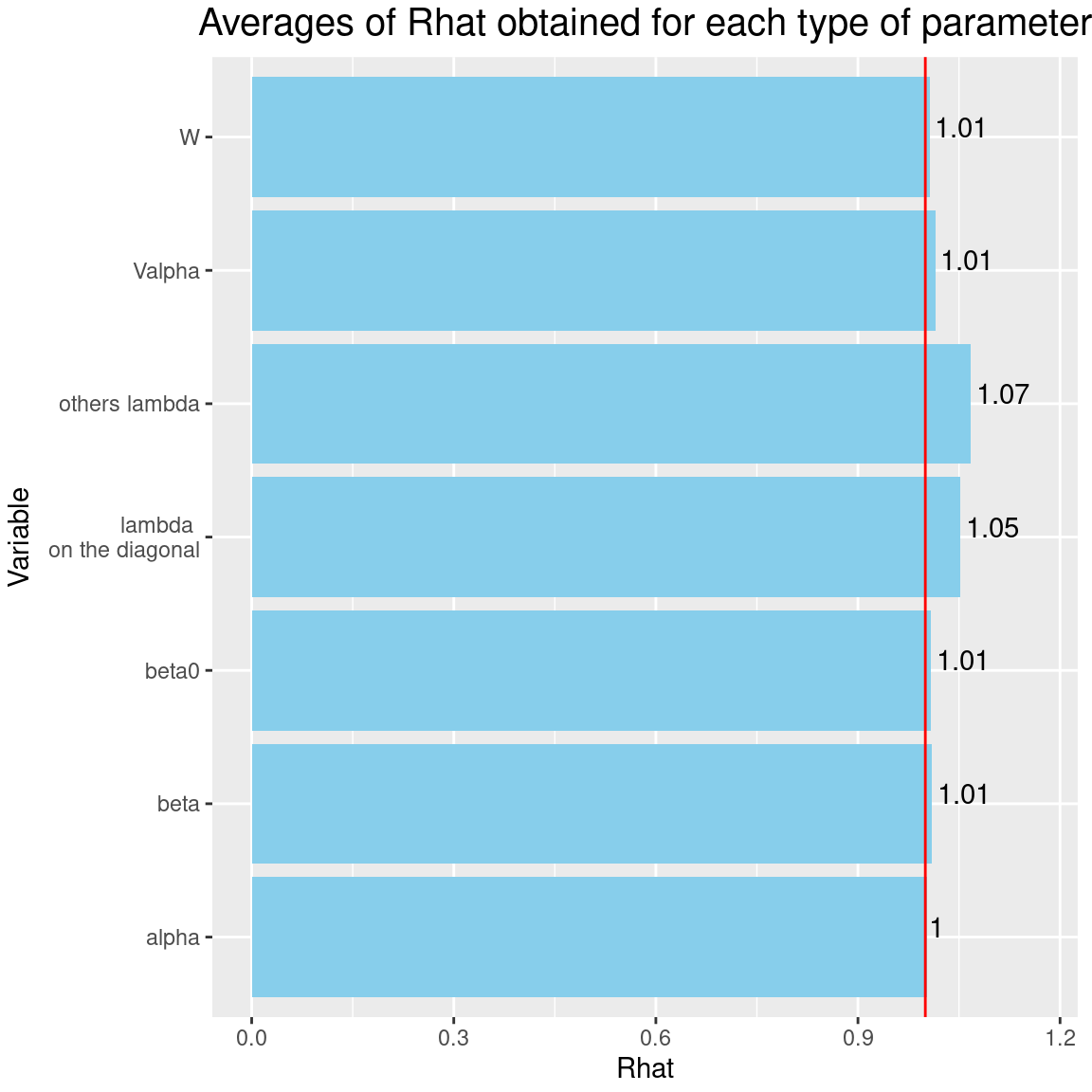
It can be seen that the \(\hat{R}\)s associated with the factor loadings \(\lambda\) are closer to 1 than previously, after the same number of iterations and with the same random number generator seeds. Consequently the convergence of factor loadings has improved by selecting the constrained species.
5.3 Analysis of the results
5.3.1 Representation of results
We visually evaluate the convergence of MCMCs by representing the trace and density a posteriori of some estimated parameters.
np <- nrow(mod_Fungi_ord[[1]]$model_spec$beta_start)
n_latent <- mod_Fungi_ord[[1]]$model_spec$n_latent
nchains <- length(mod_Fungi_ord)
## beta_j of the first two species and the species constrained
par(mfrow=c(2,2))
plot(mcmc_list_beta[,1:(2*np)],
auto.layout=FALSE)
for(l in 1:n_latent){
par(mfrow=c(2,2))
plot(mcmc_list_beta[, grep(mod_Fungi_ord[[1]]$sp_constrained[l],
varnames(mcmc_list_beta))],
auto.layout=FALSE)
}
## lambda_j of the first two species and the species constrained
par(mfrow=c(2,2))
plot(mcmc_list_lambda[,1:(2*n_latent)],
auto.layout=FALSE)
for(l in 1:n_latent){
par(mfrow=c(2,2))
plot(mcmc_list_lambda[, grep(mod_Fungi_ord[[1]]$sp_constrained[l],
varnames(mcmc_list_lambda))],
auto.layout=FALSE)
}
## Latent variables W_i for the first two sites
par(mfrow=c(2,2))
nsite <- ncol(mod_Fungi_ord[[1]]$mcmc.alpha)
plot(mcmc_list_lv[, c(1:2, 1:2 + nsite)],
auto.layout=FALSE)
## alpha_i of the first two sites
plot(mcmc_list_alpha[,1:2])
## V_alpha
par(mfrow=c(2,2))
plot(mcmc_list_V_alpha,
auto.layout=FALSE)
## Deviance
plot(mcmc_list_Deviance,
auto.layout=FALSE)
## probit_theta
par(mfrow=c(1,2))
hist(mod_Fungi_ord[[1]]$probit_theta_latent,
col=1,
main = "Predicted probit theta",
xlab ="predicted probit theta")
for(i in 2:nchains){
hist(mod_Fungi_ord[[i]]$probit_theta_latent,
add=TRUE, col=i)
}
# occurrence probabilities theta
hist(mod_Fungi_ord[[1]]$theta_latent,
col=1, main = "Predicted theta",
xlab ="predicted theta")
for(i in 2:nchains){
hist(mod_Fungi_ord[[i]]$theta_latent,
add=TRUE, col=i)
}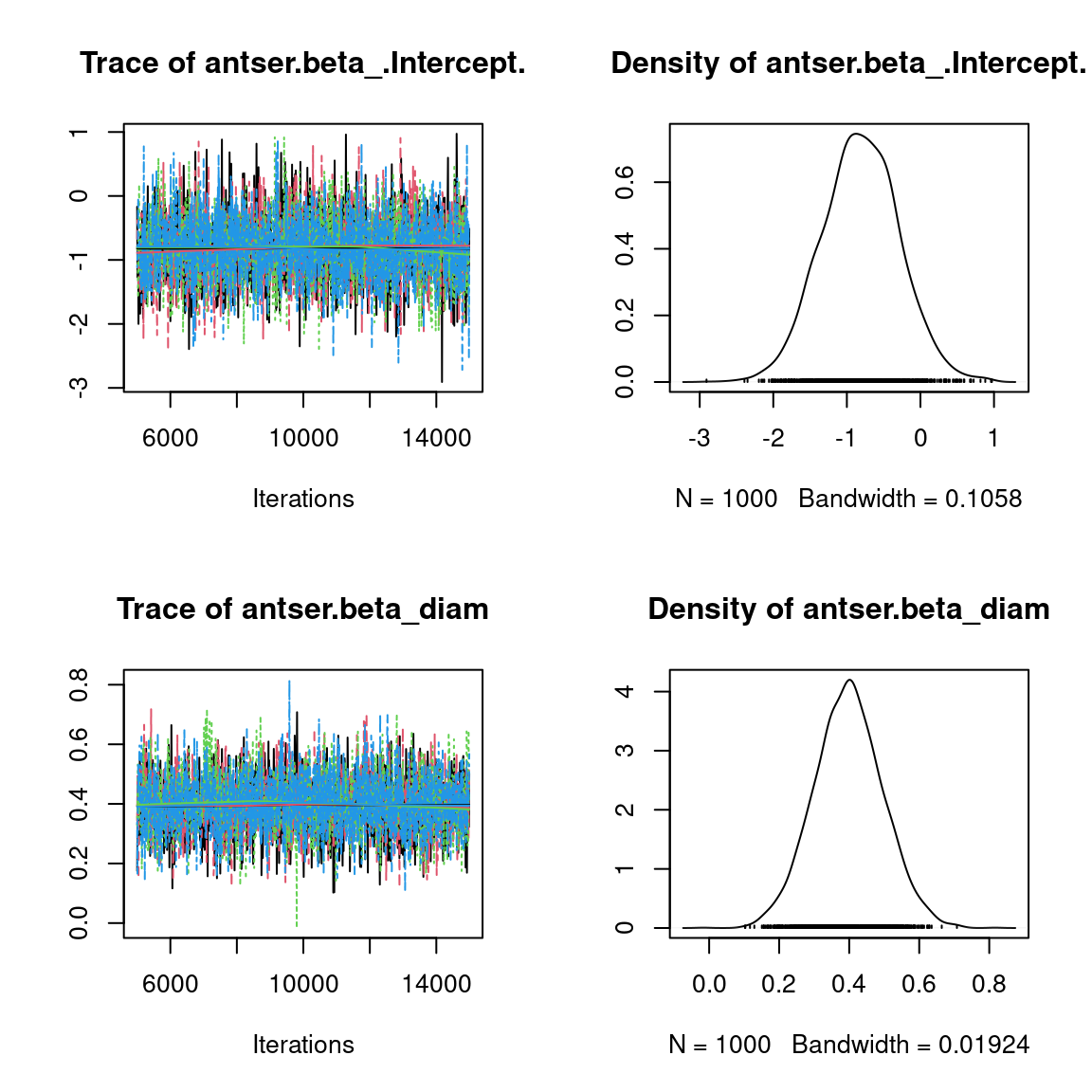
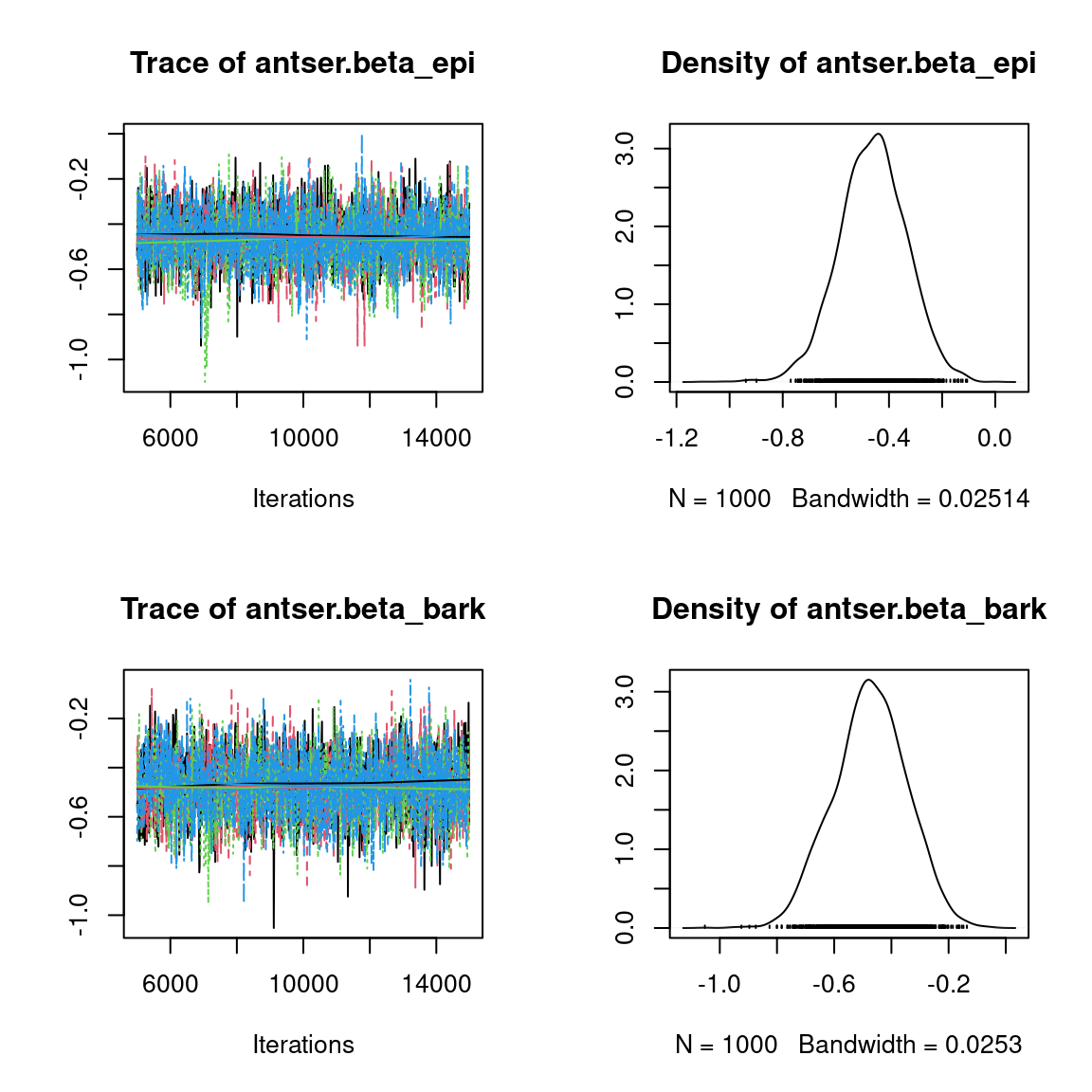
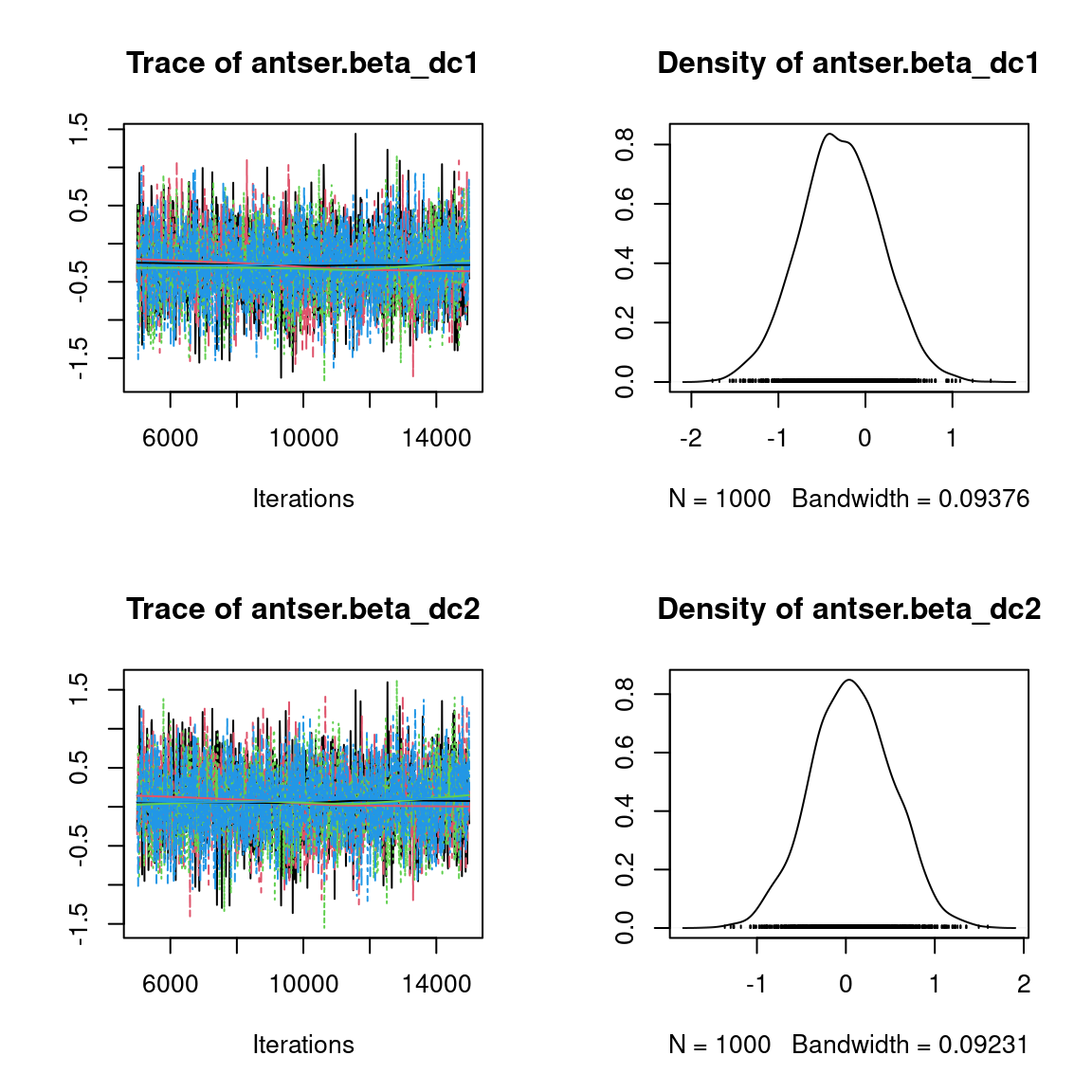
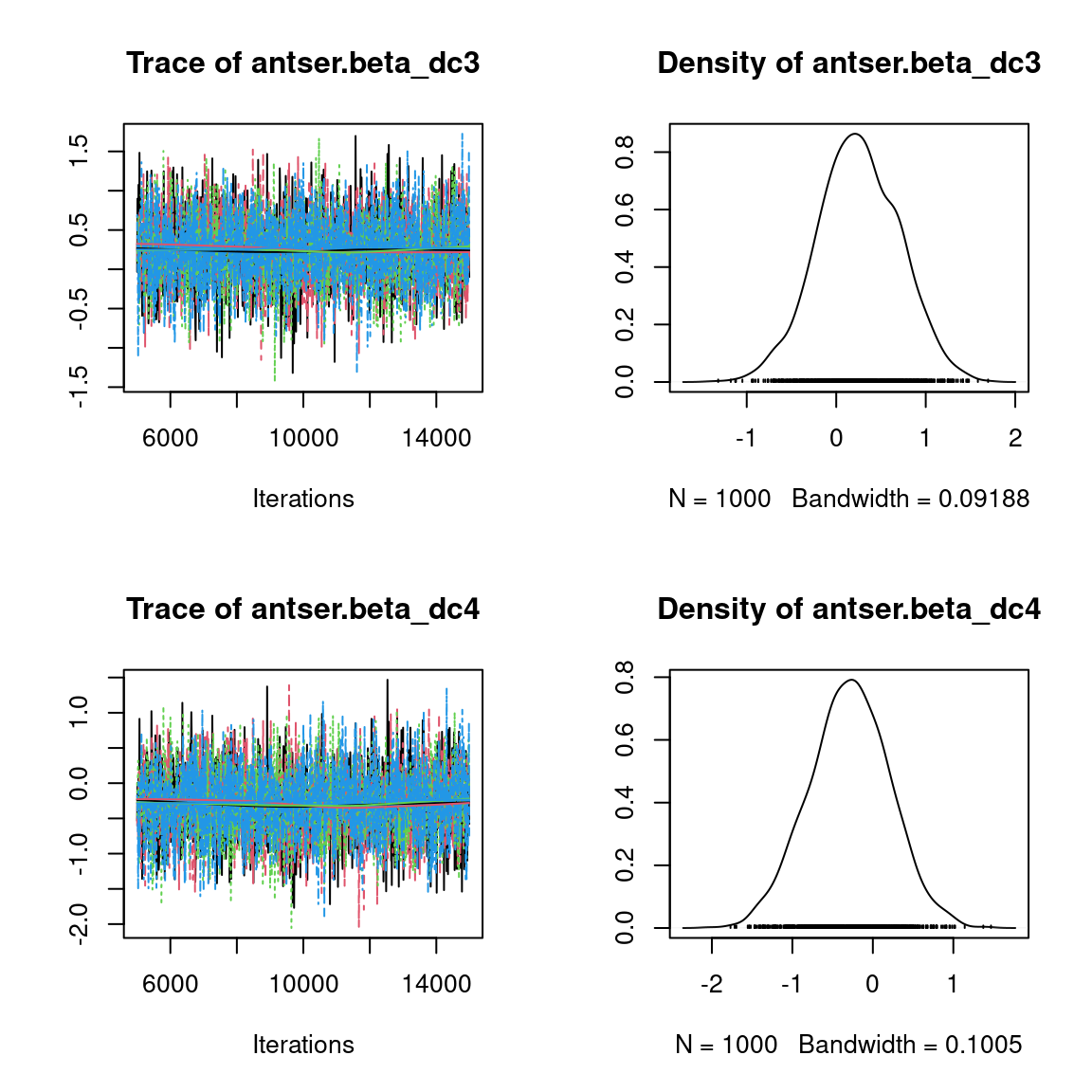
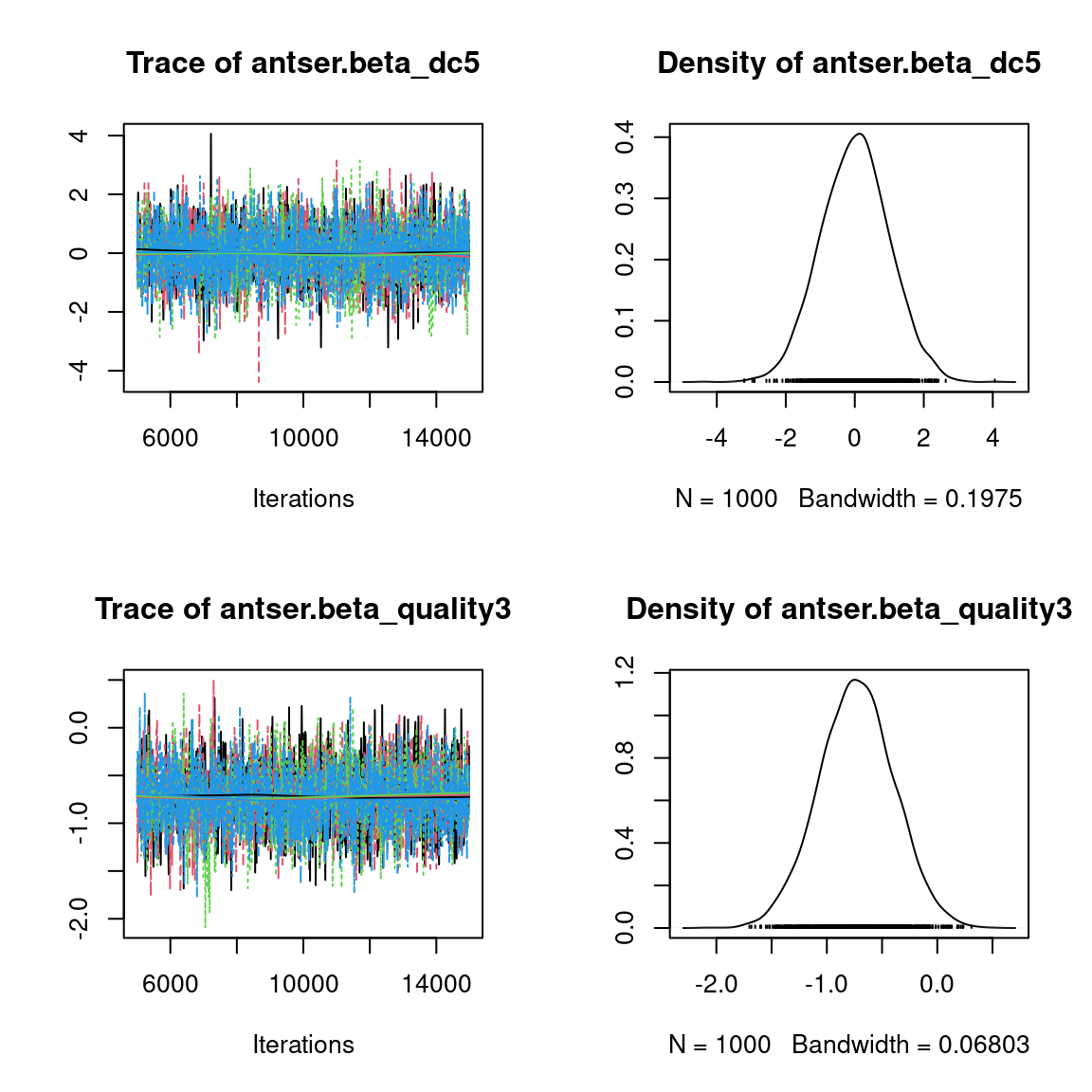
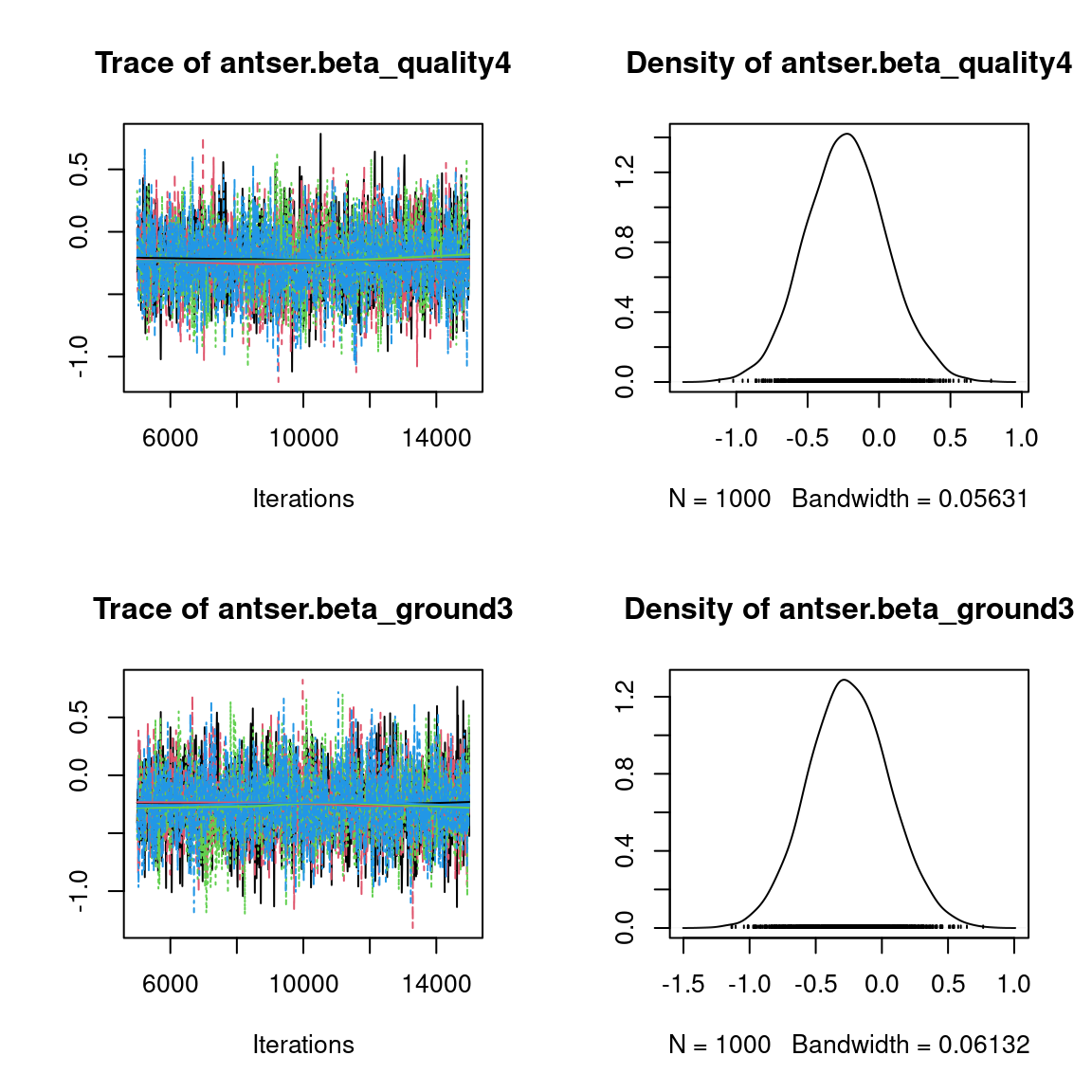
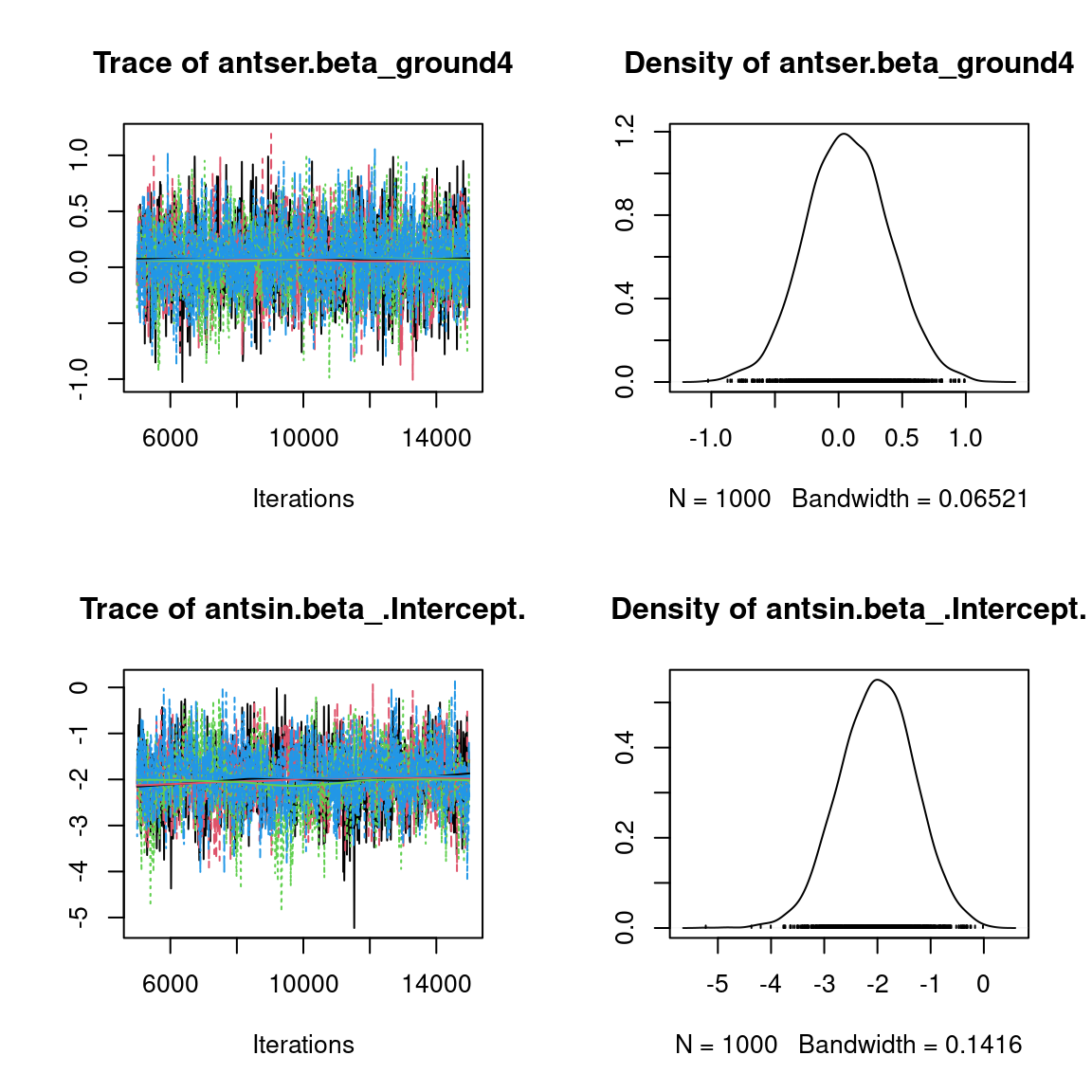
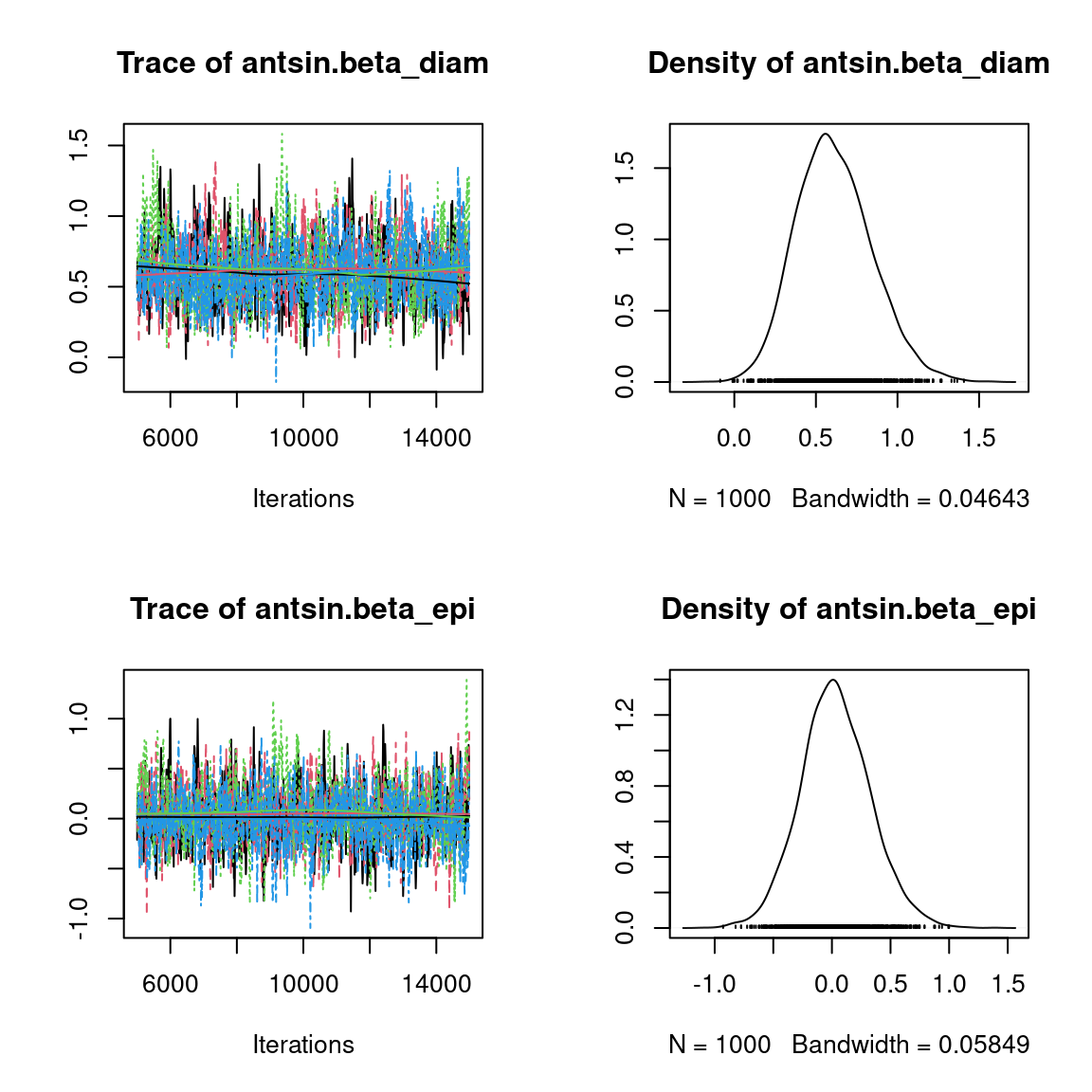
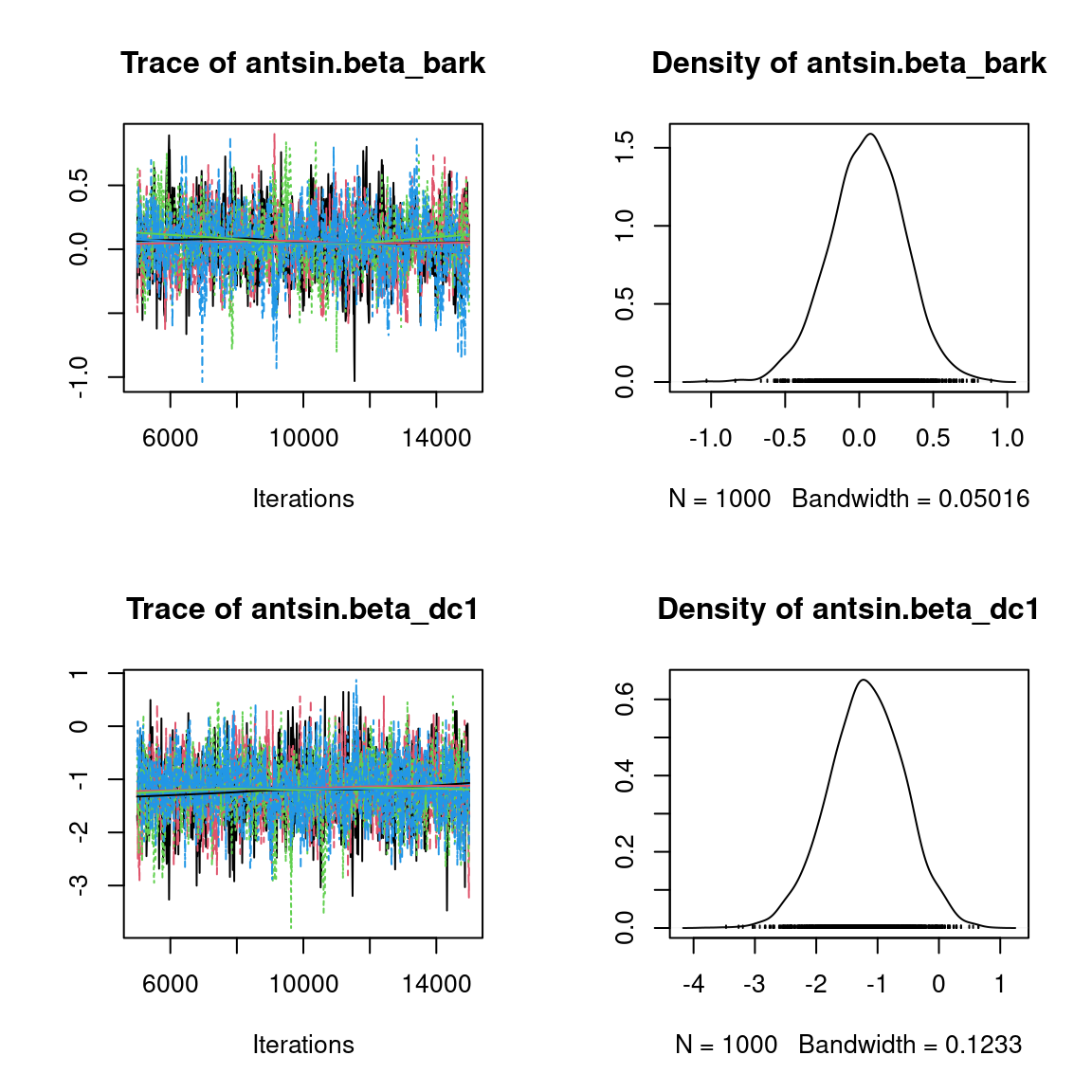
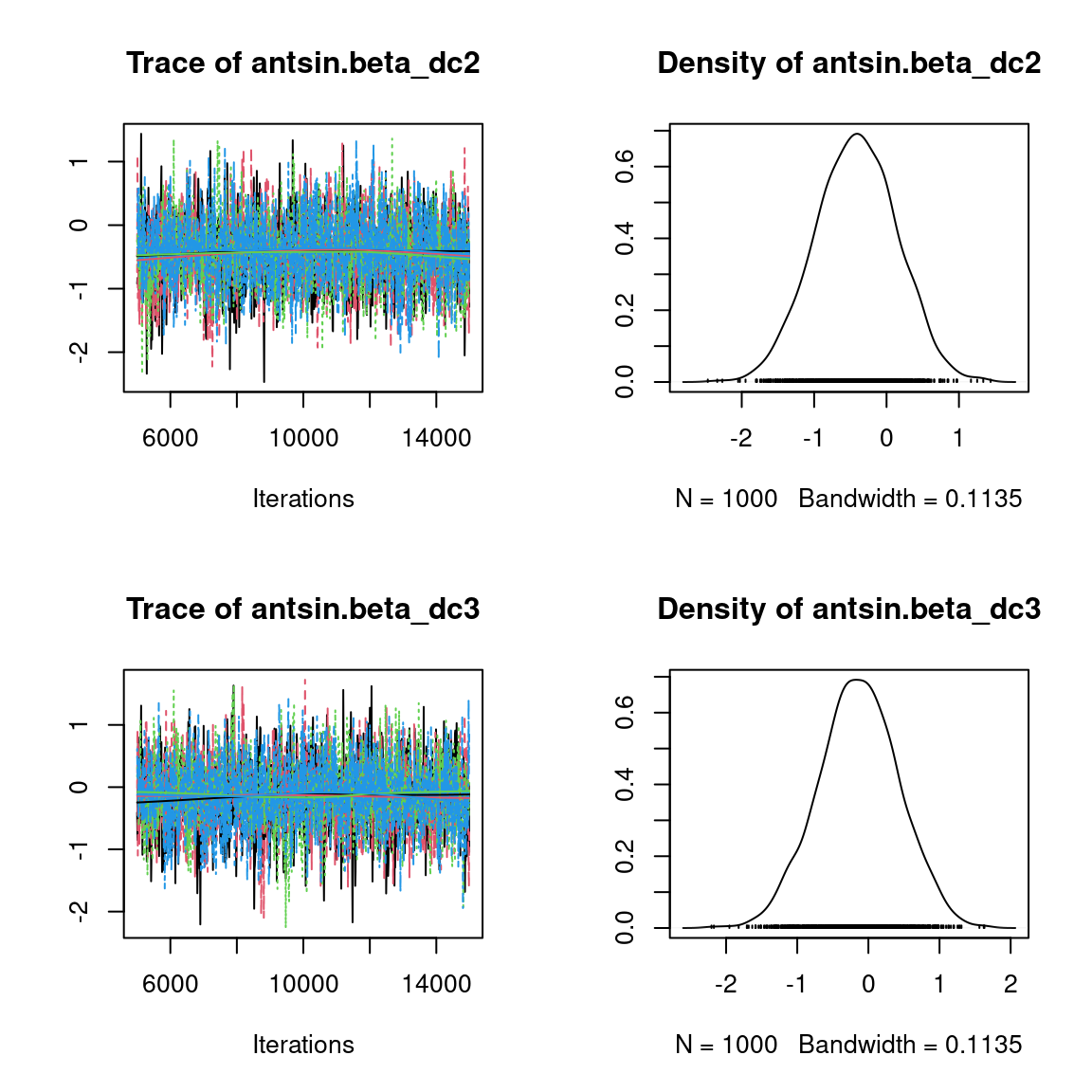
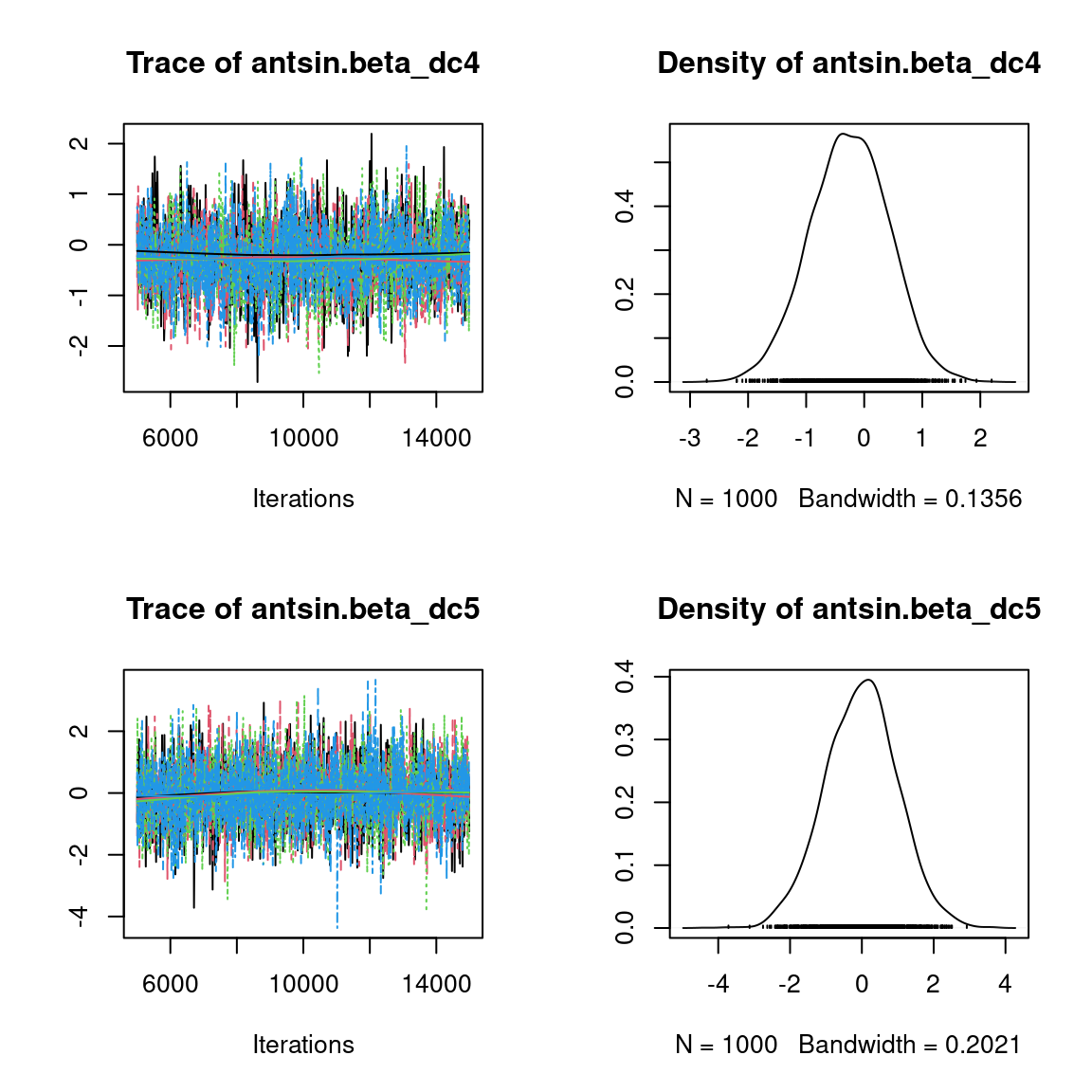
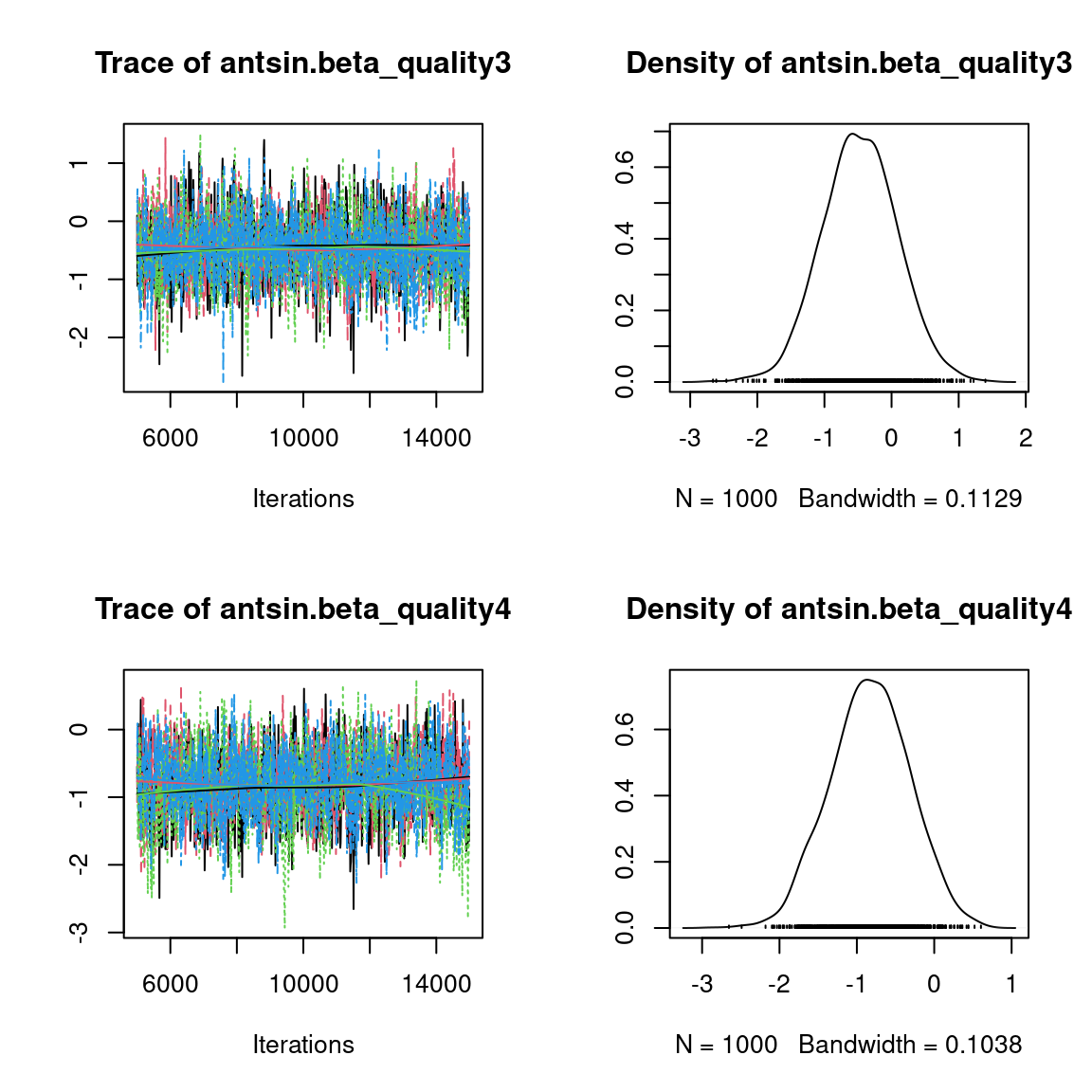
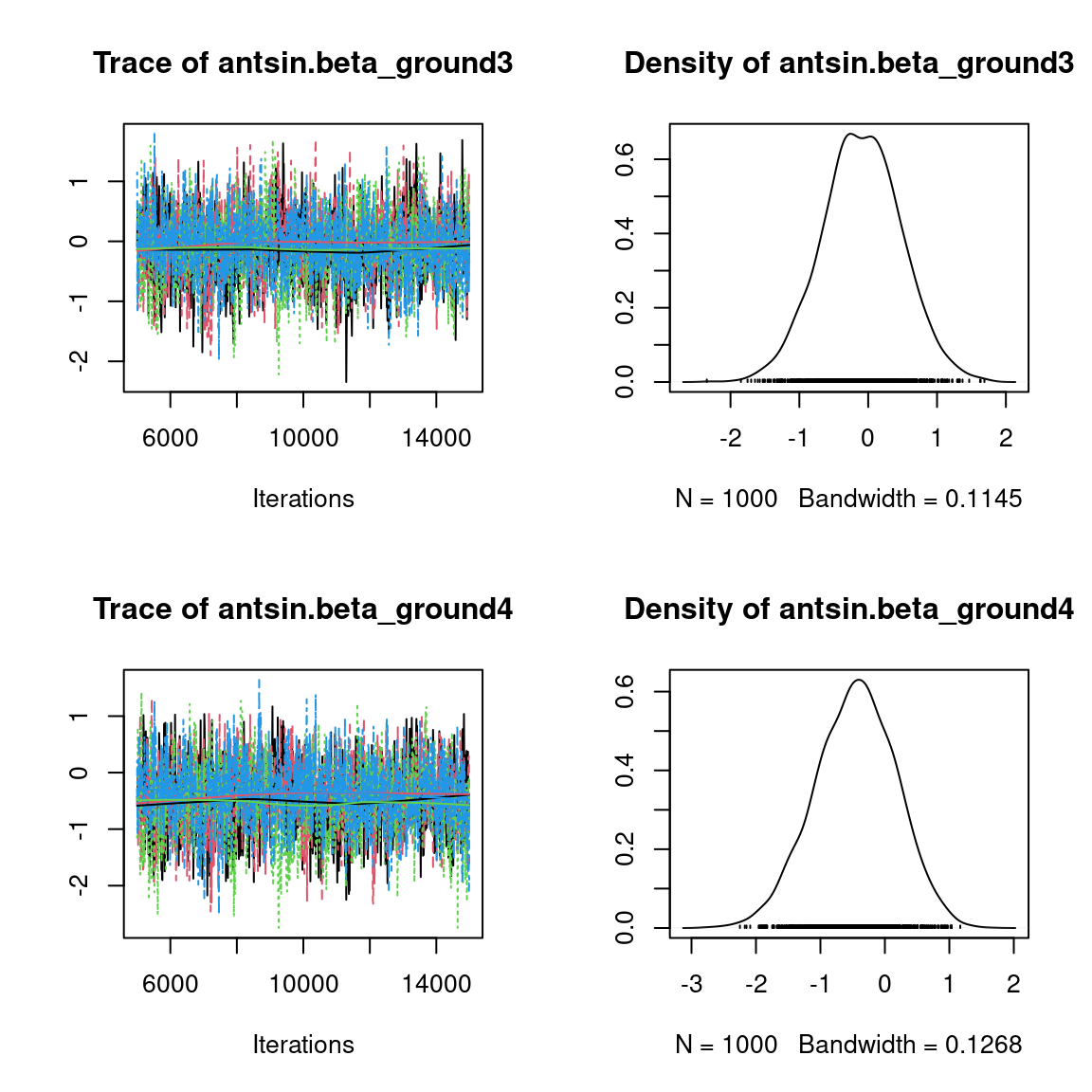
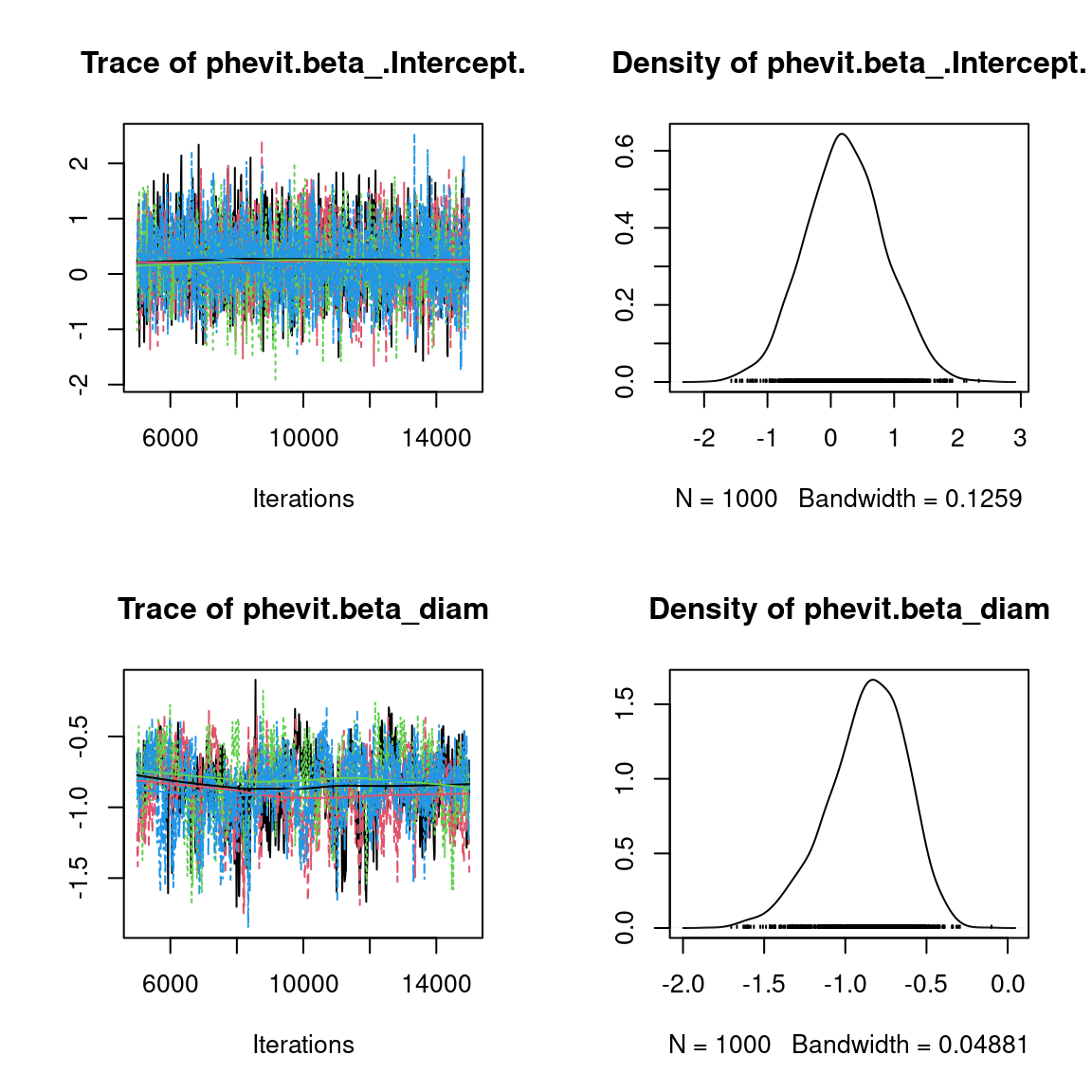
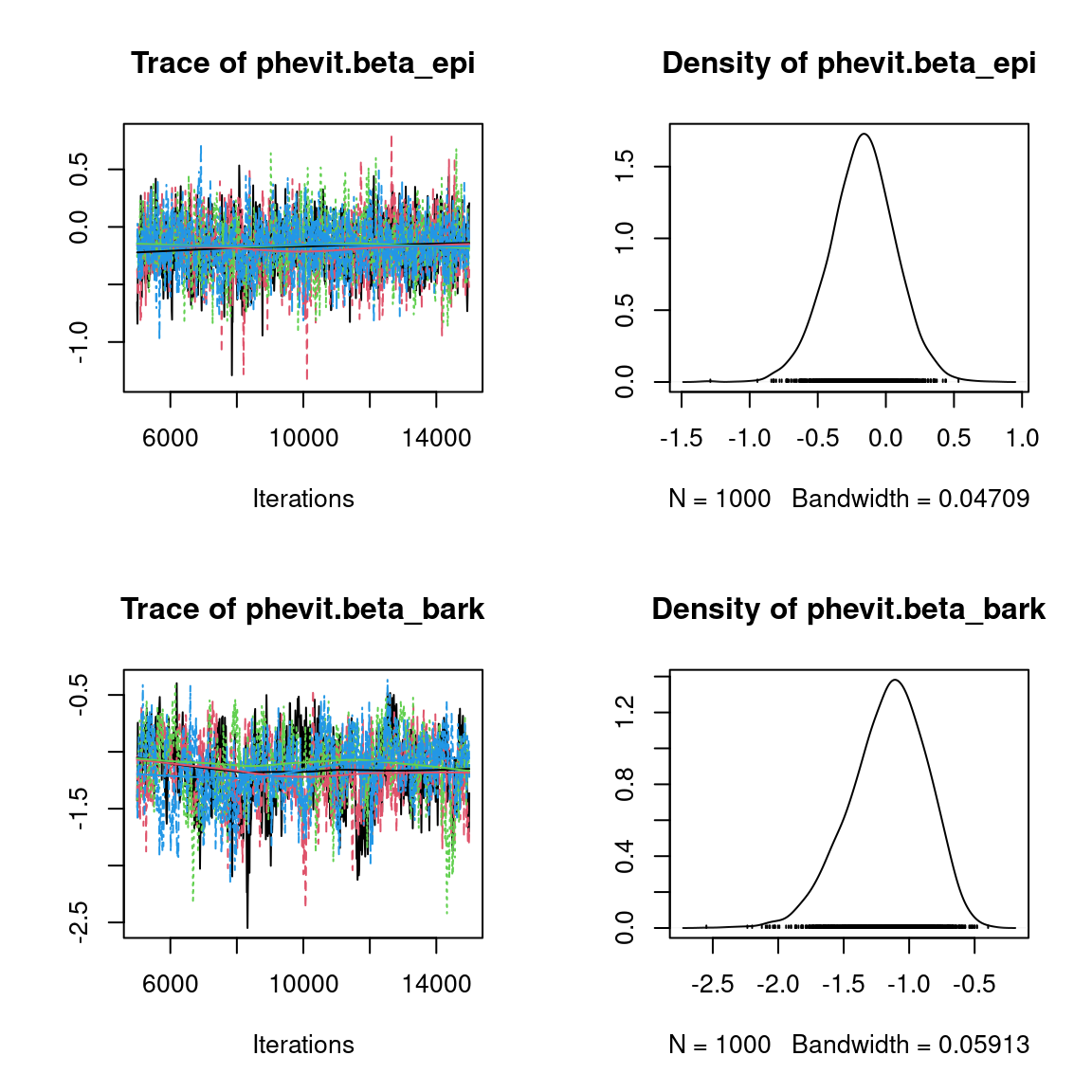
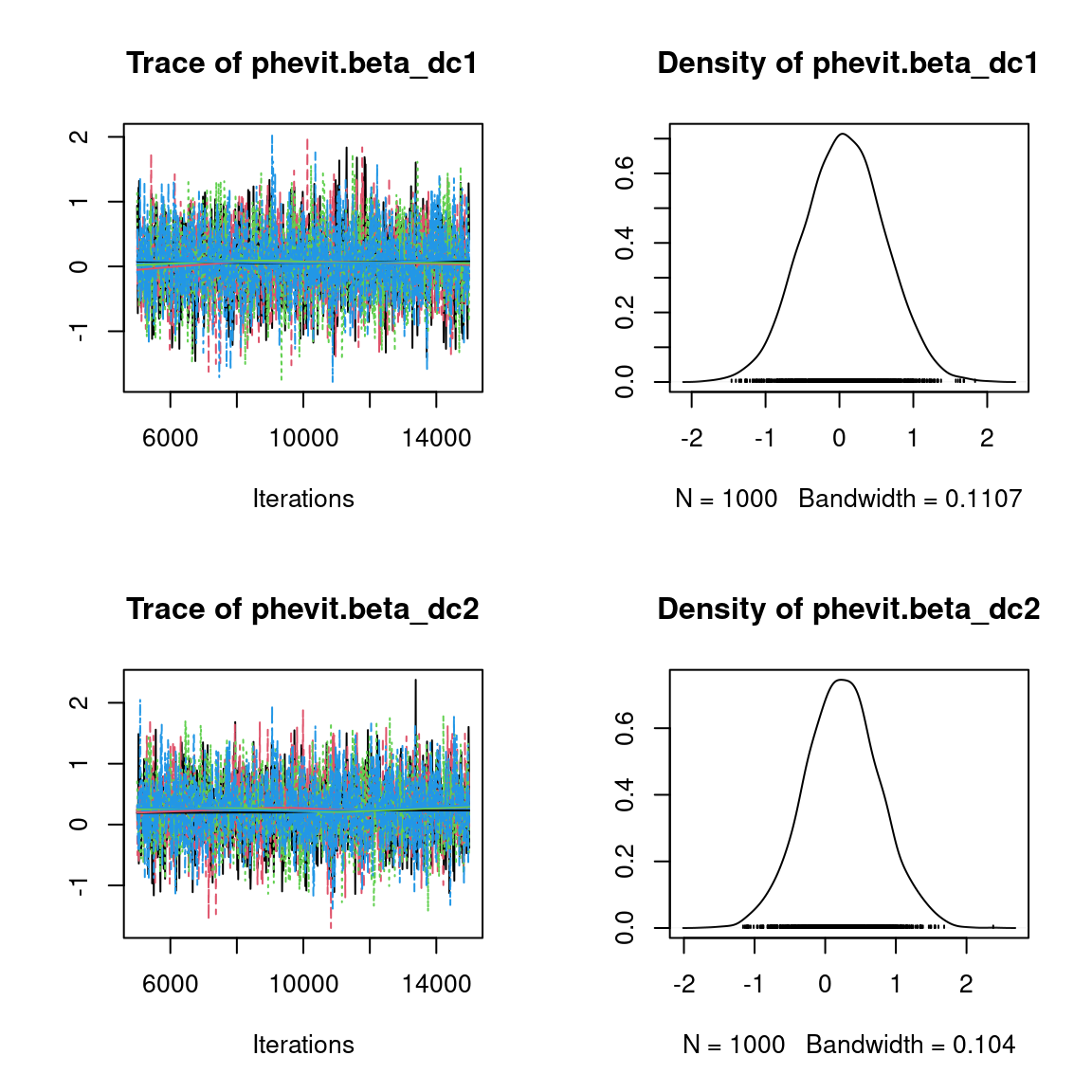
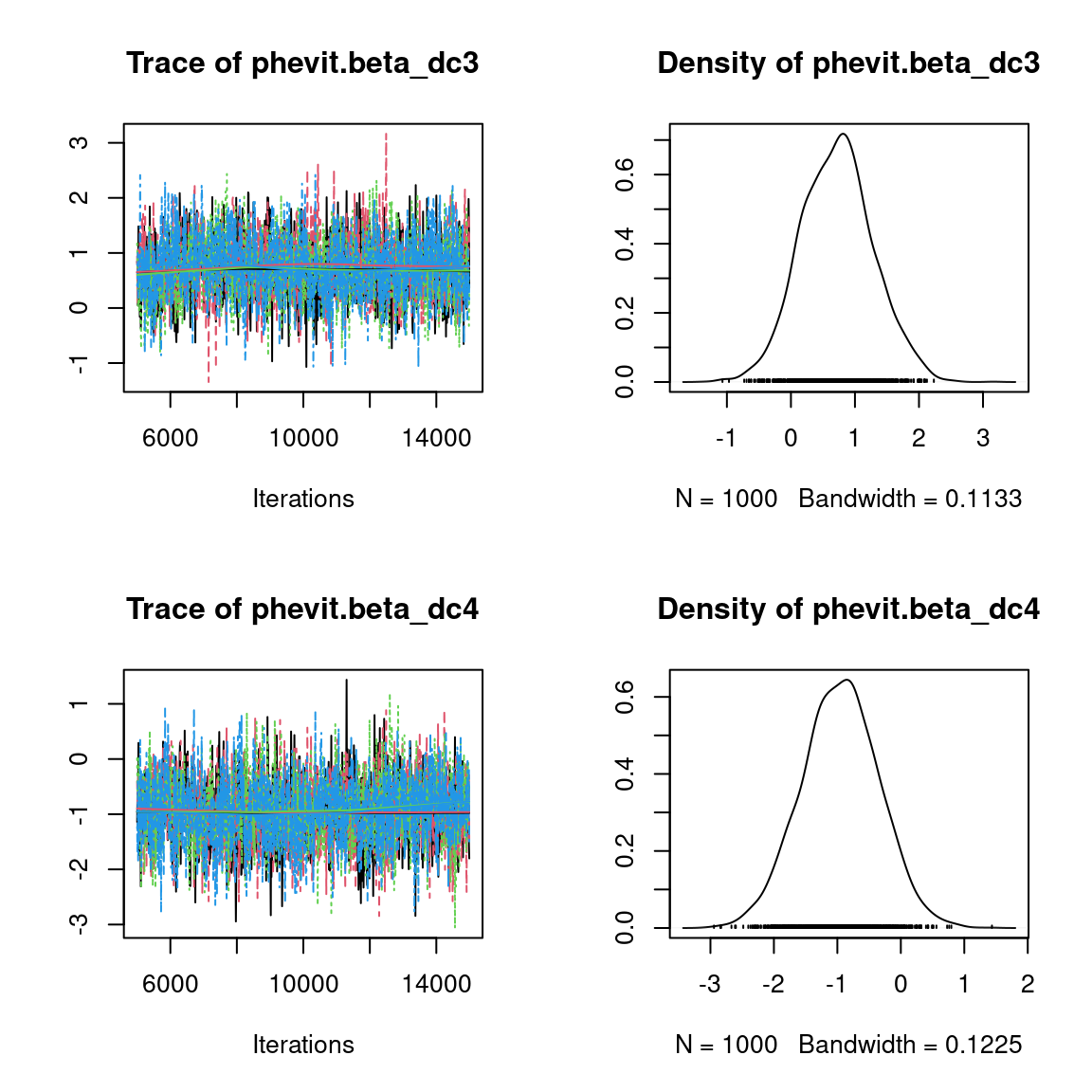
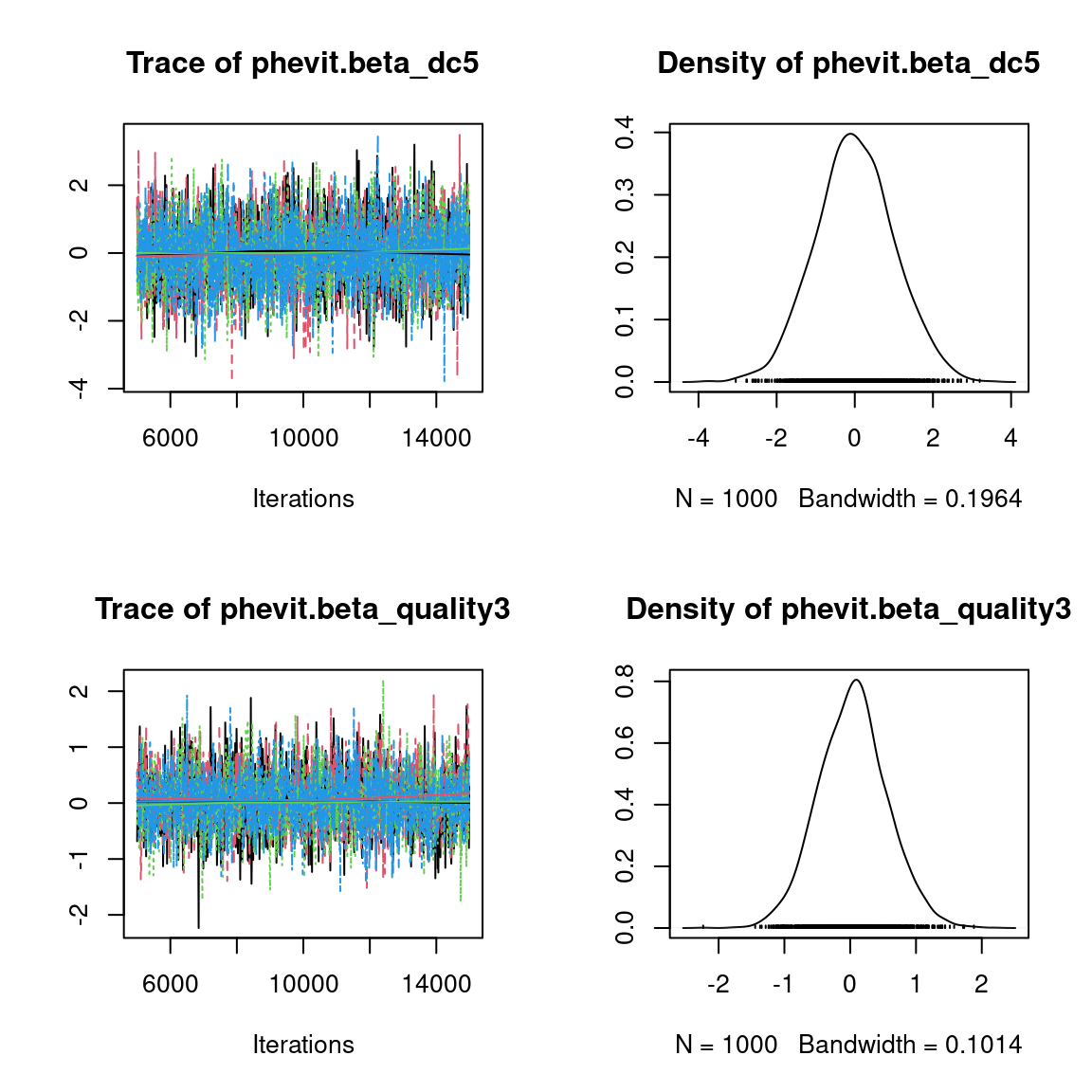
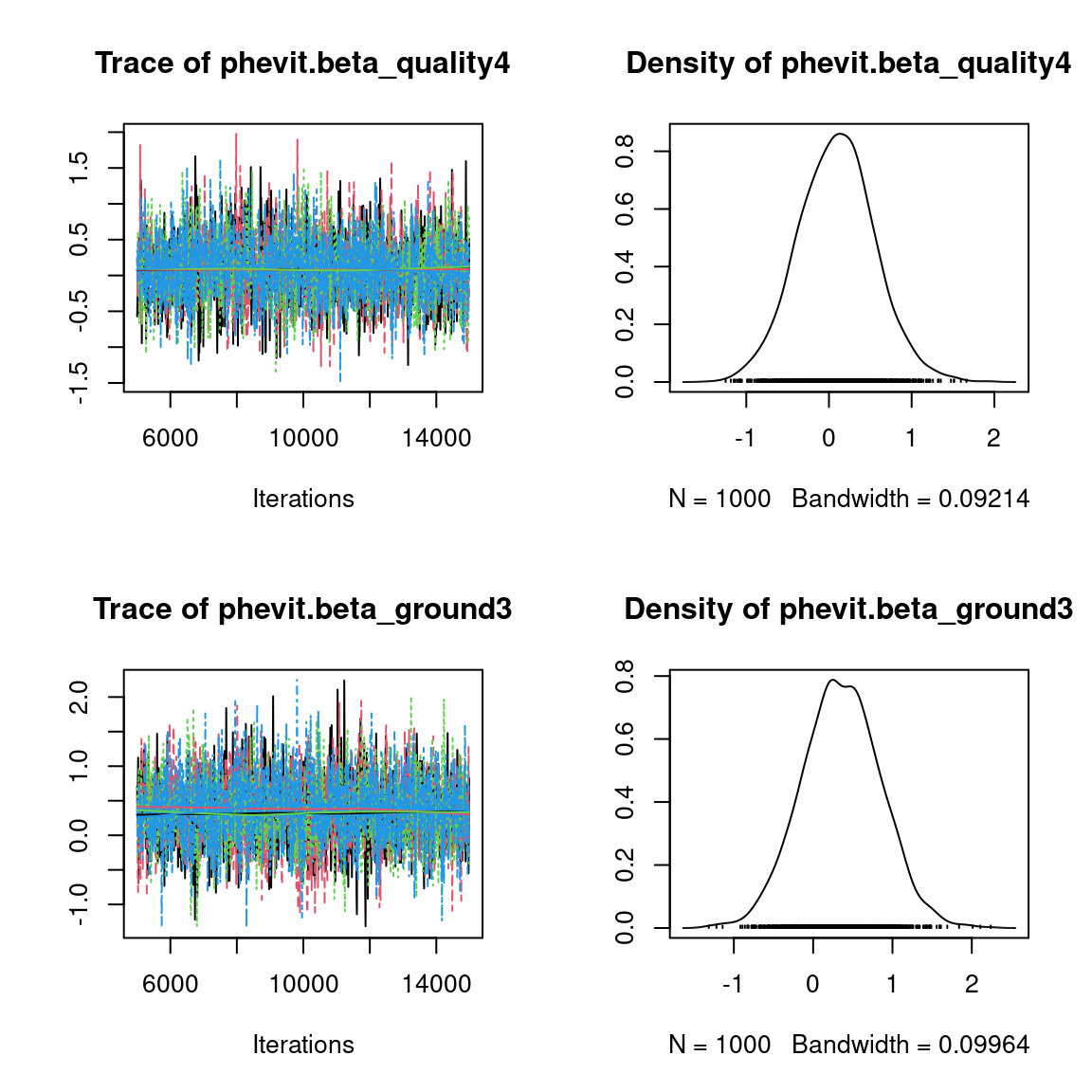
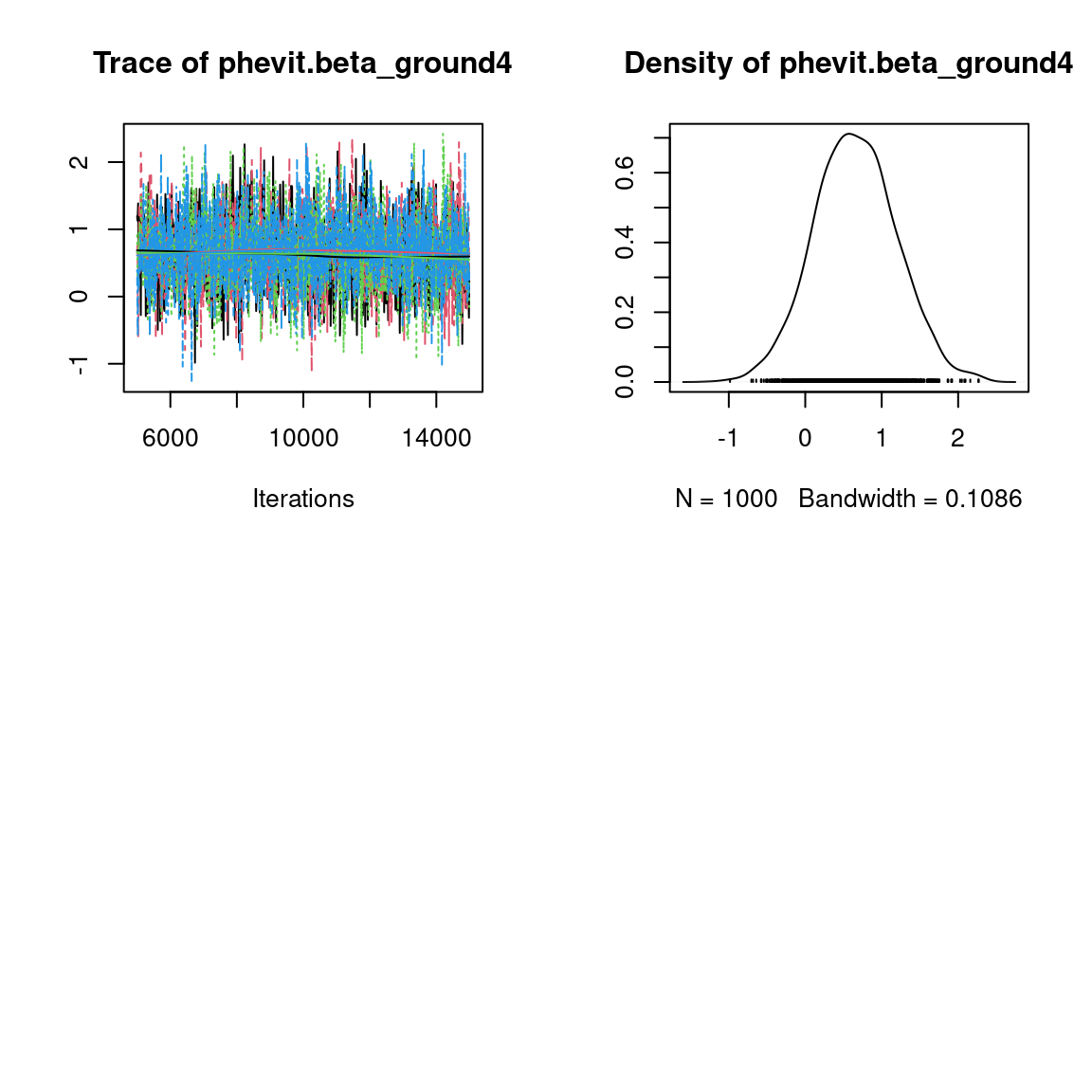
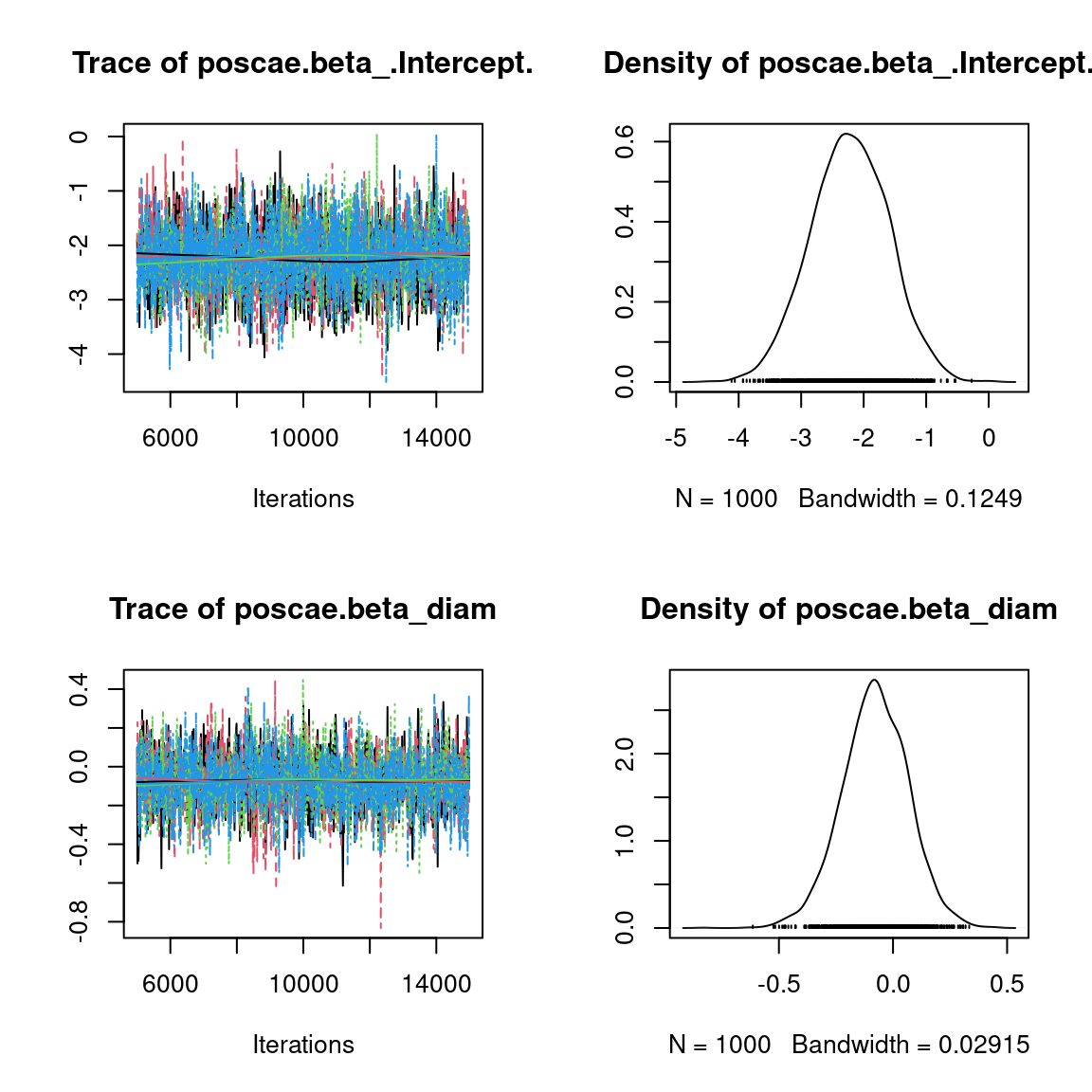
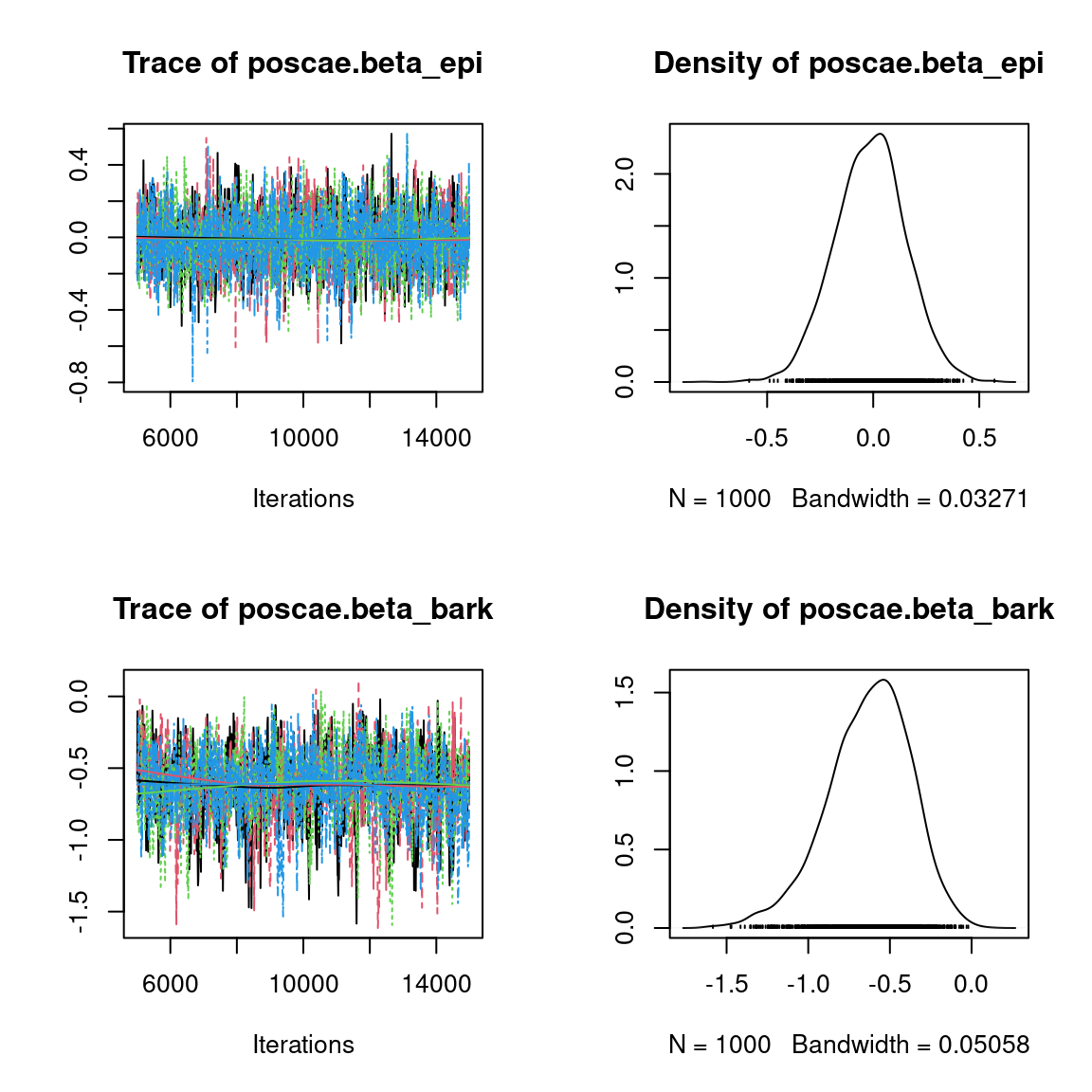
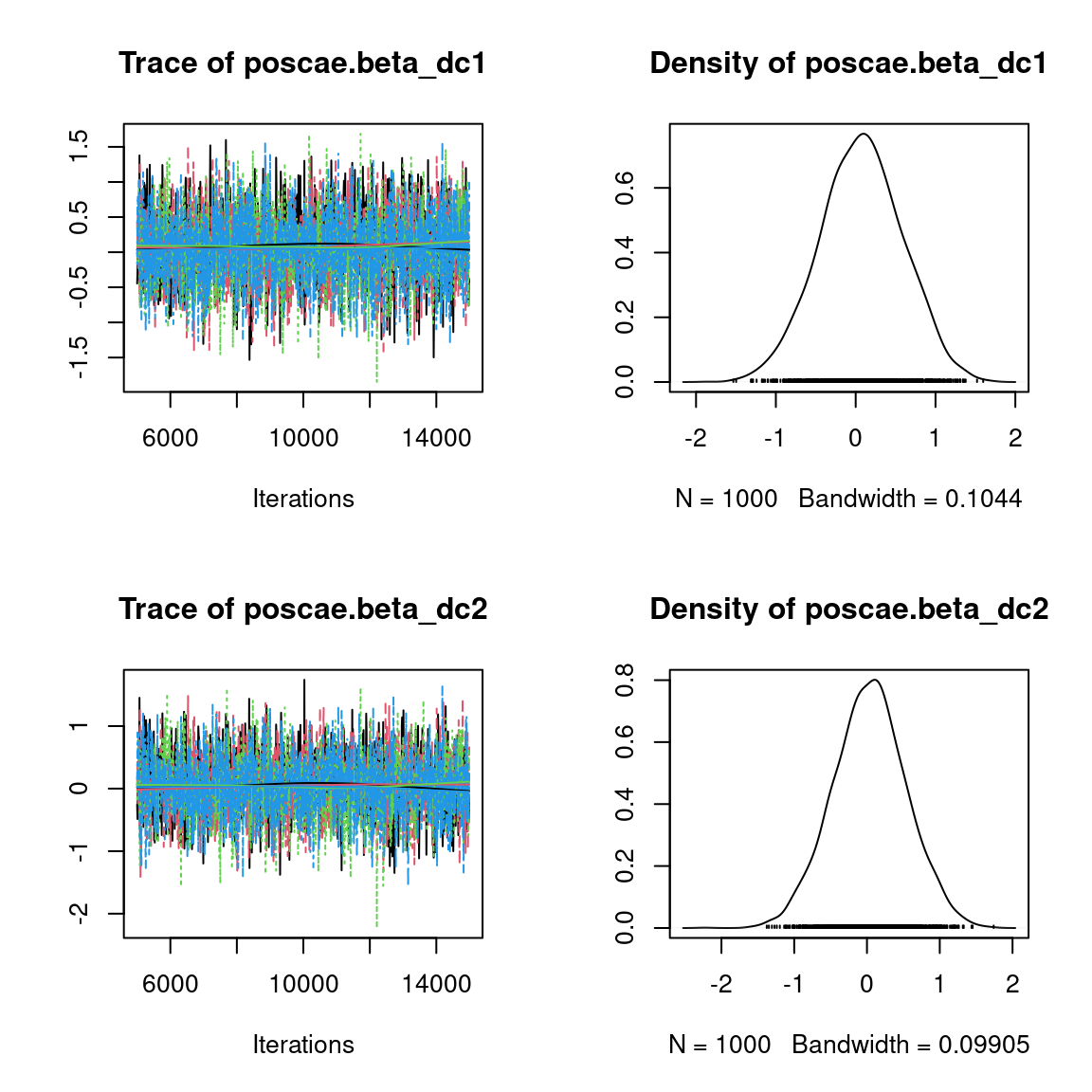
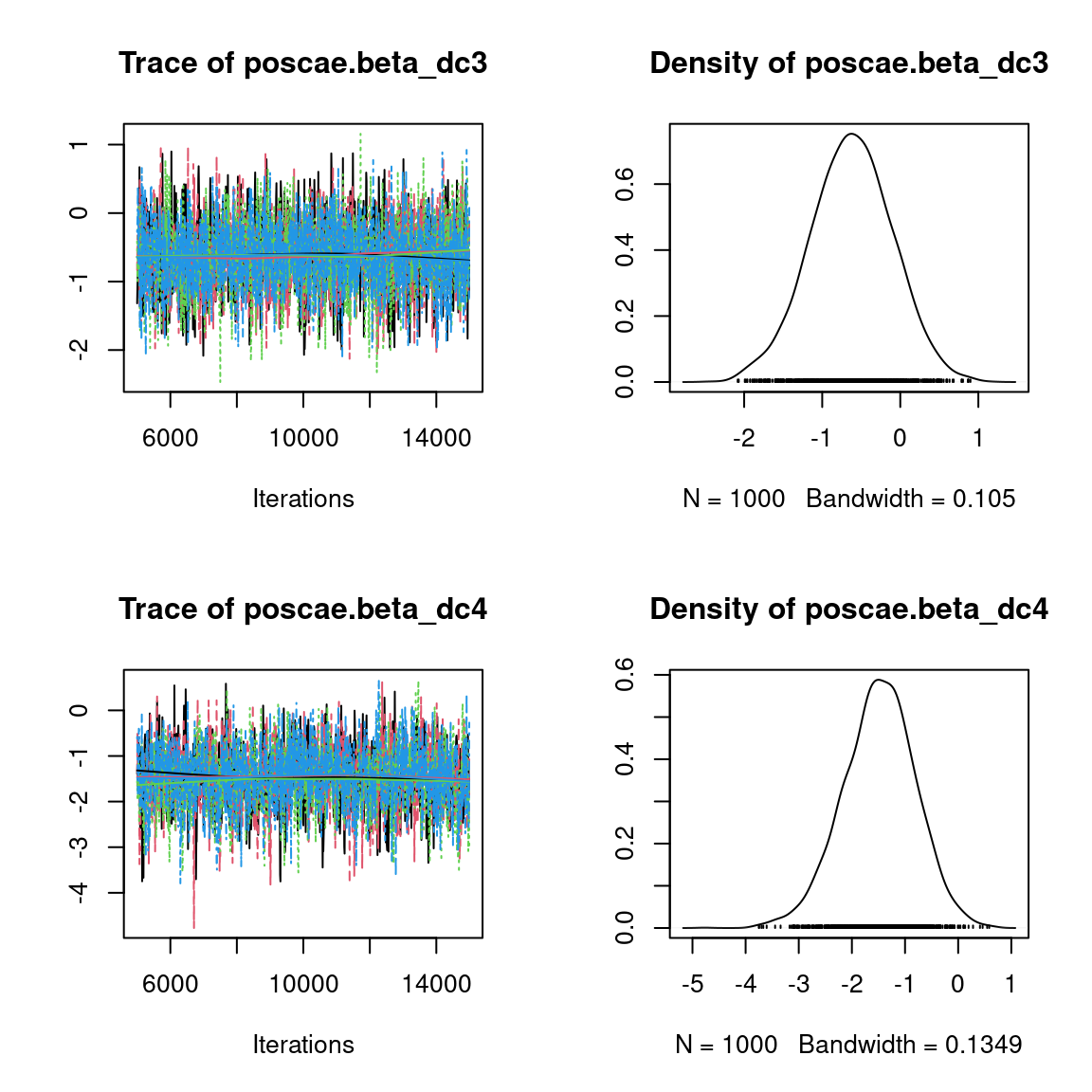
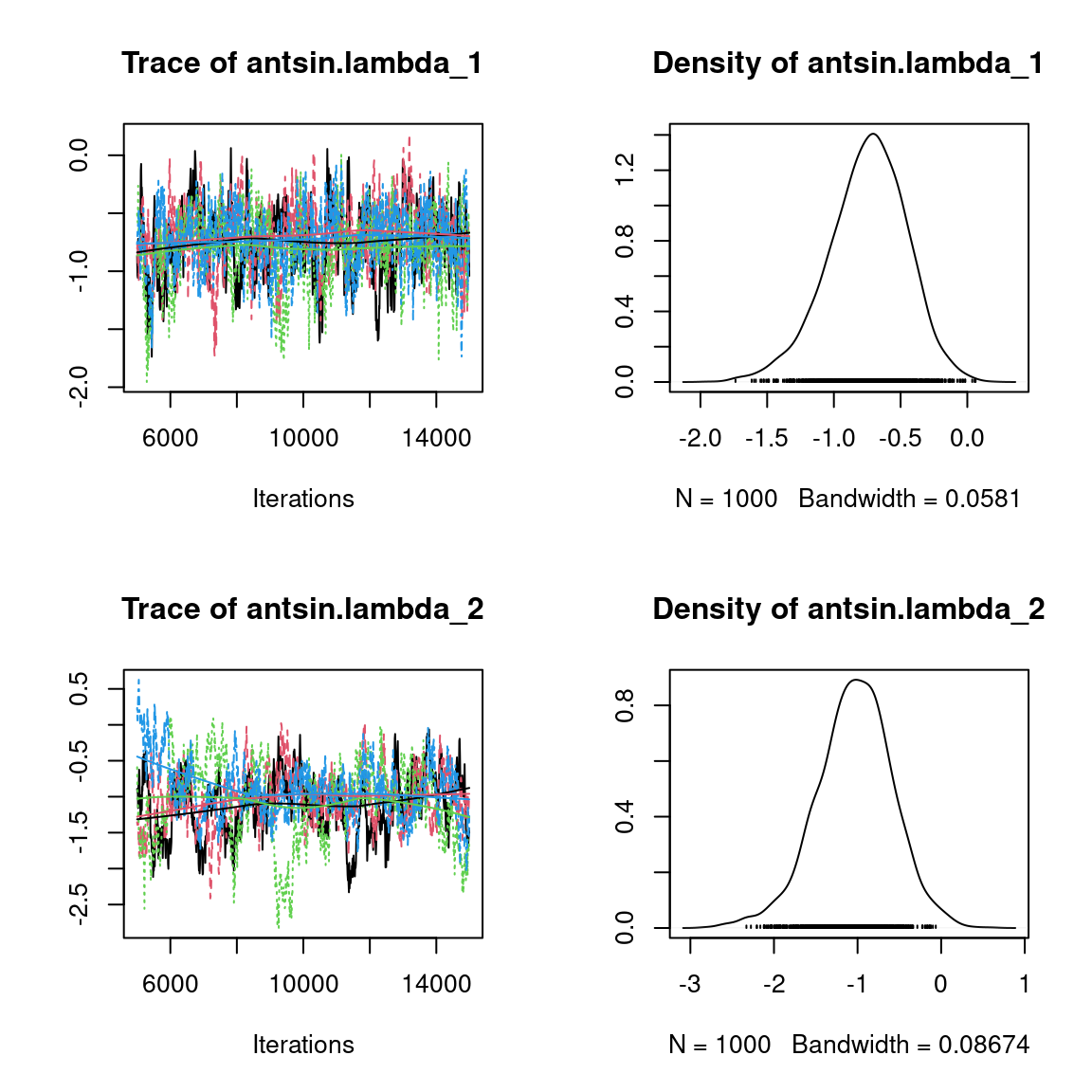
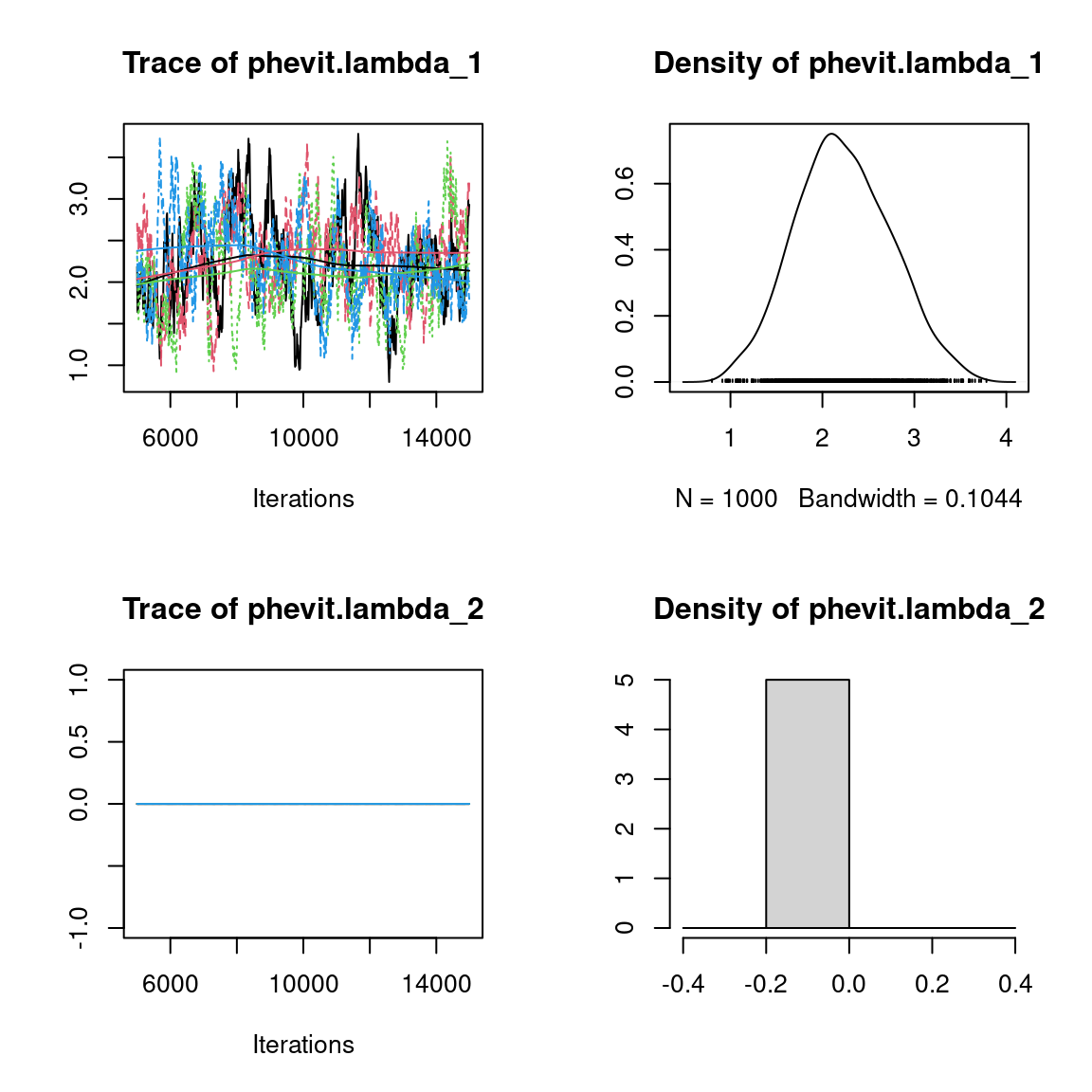
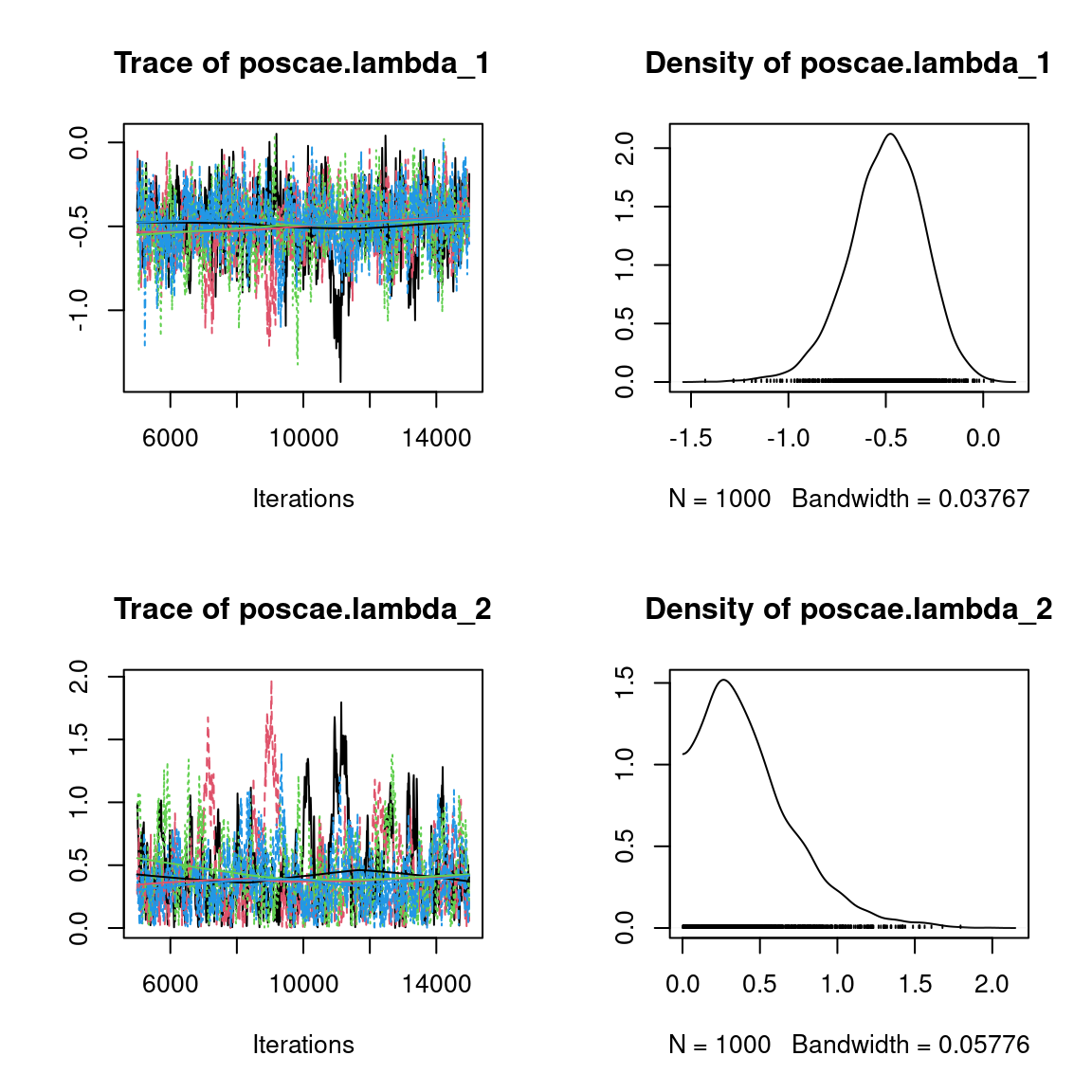
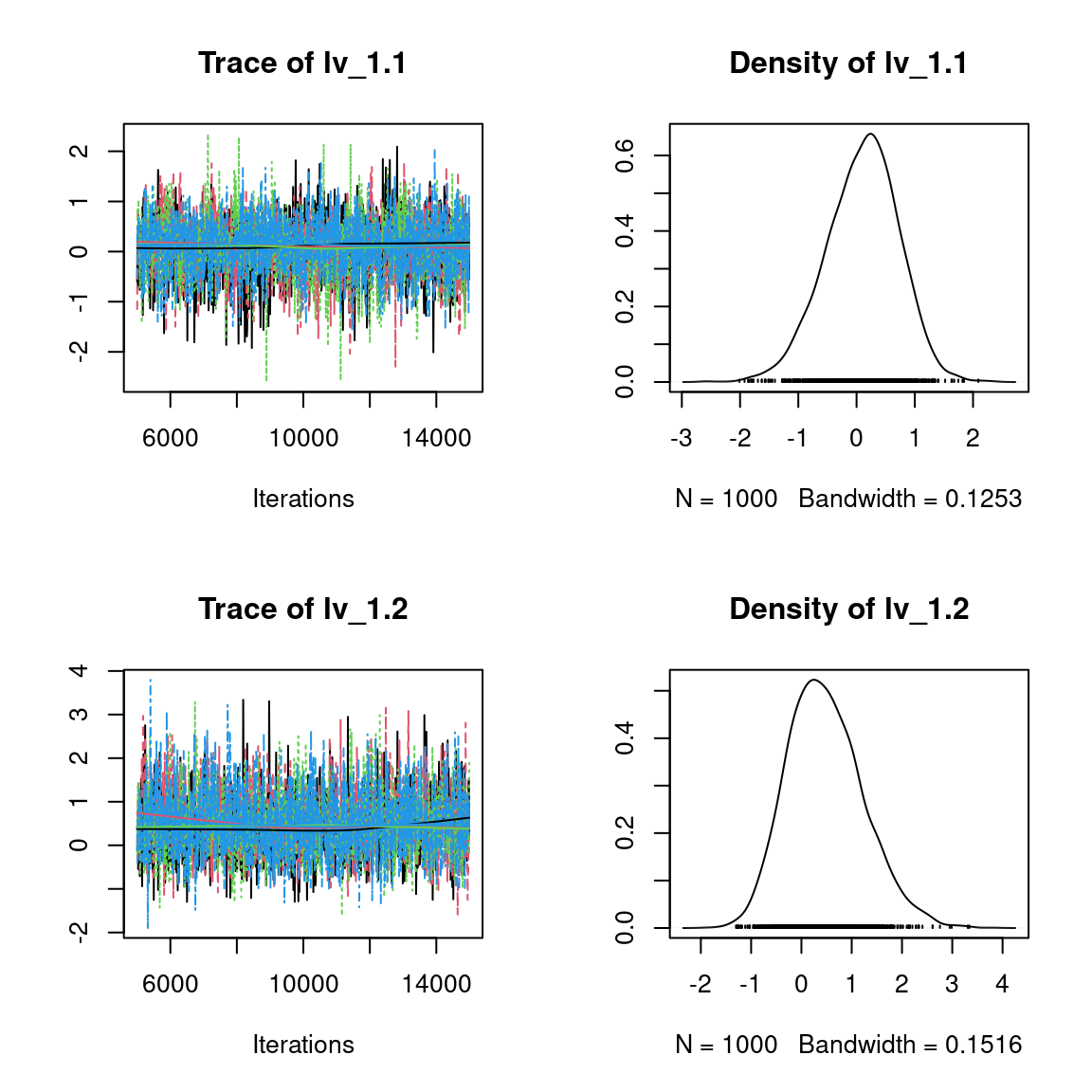
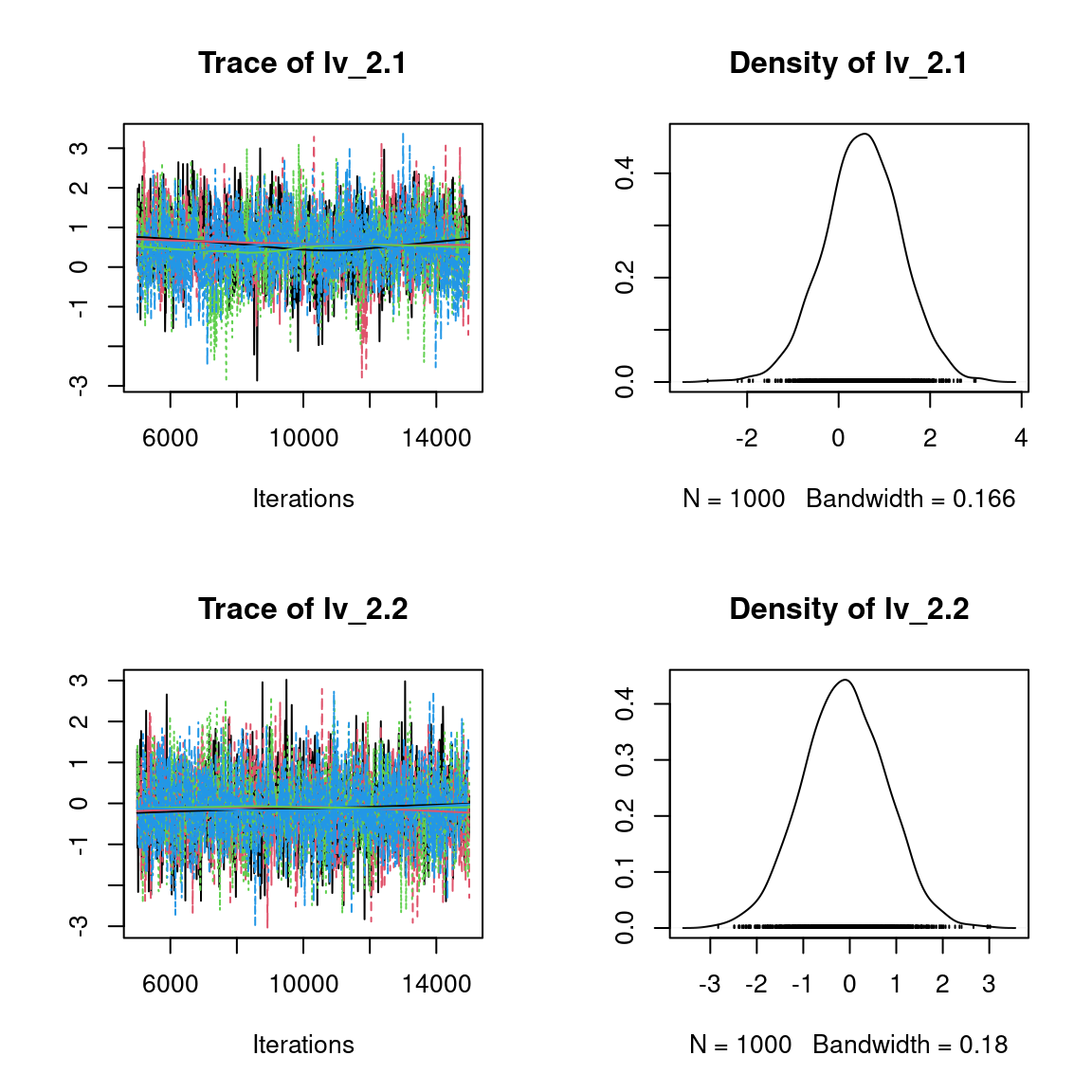
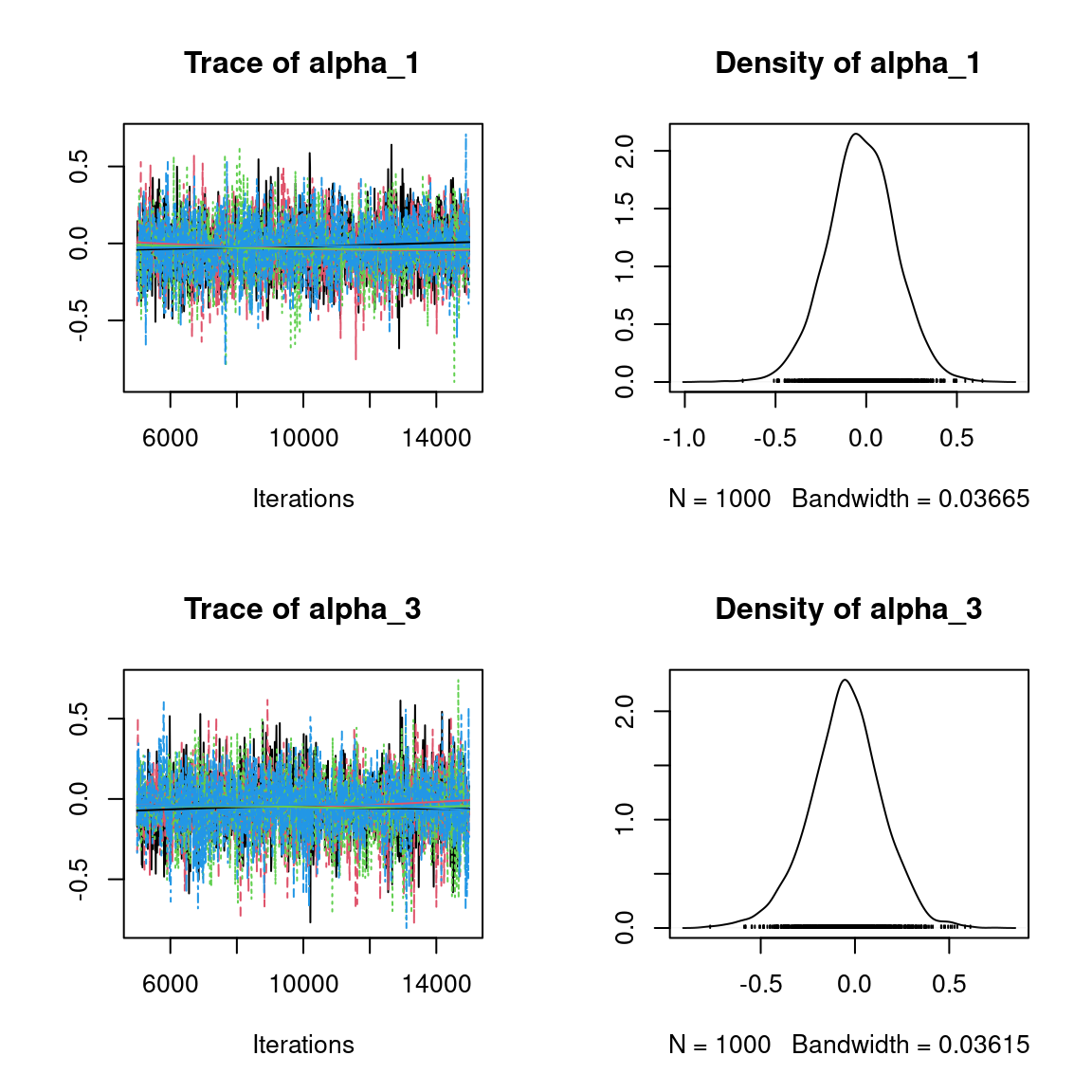
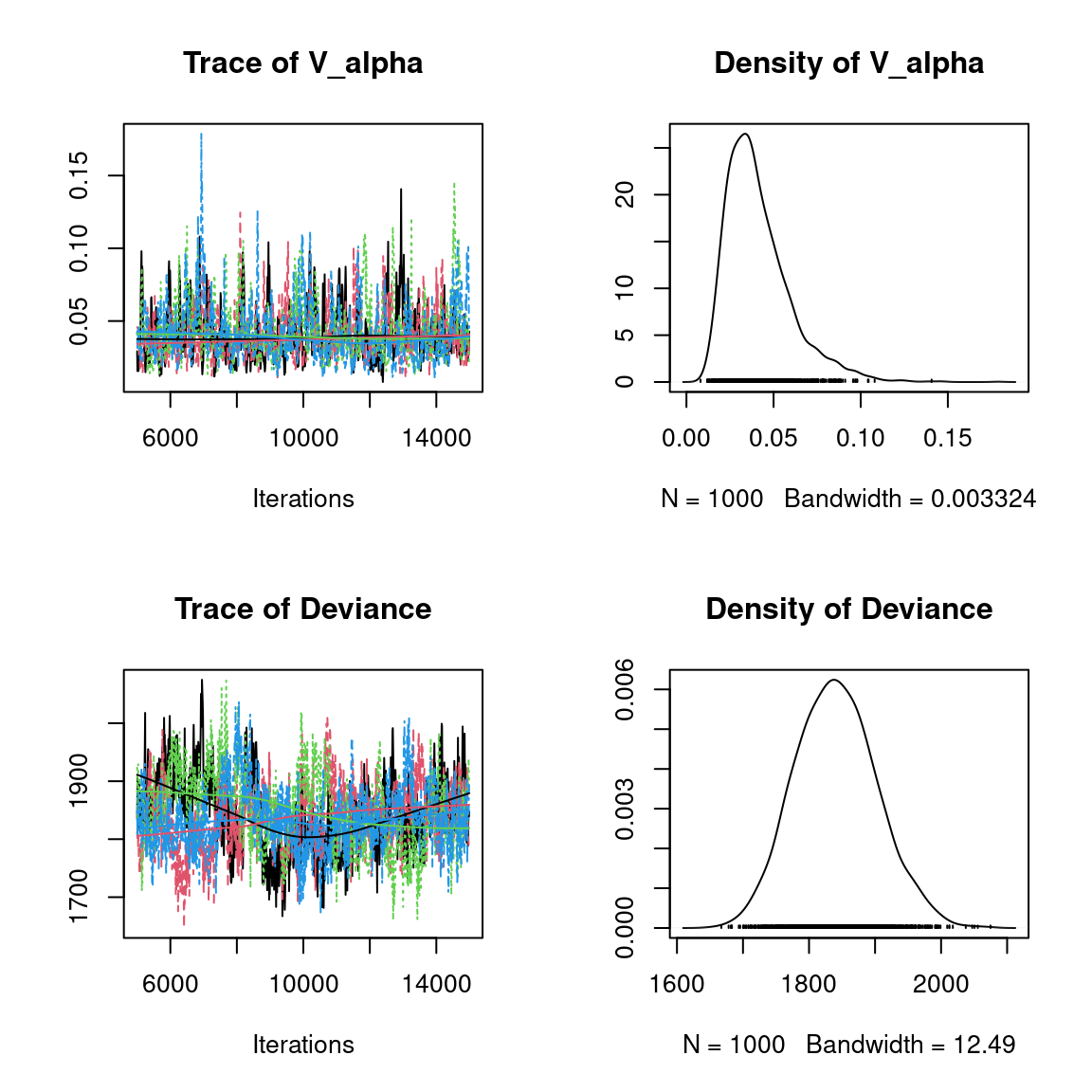
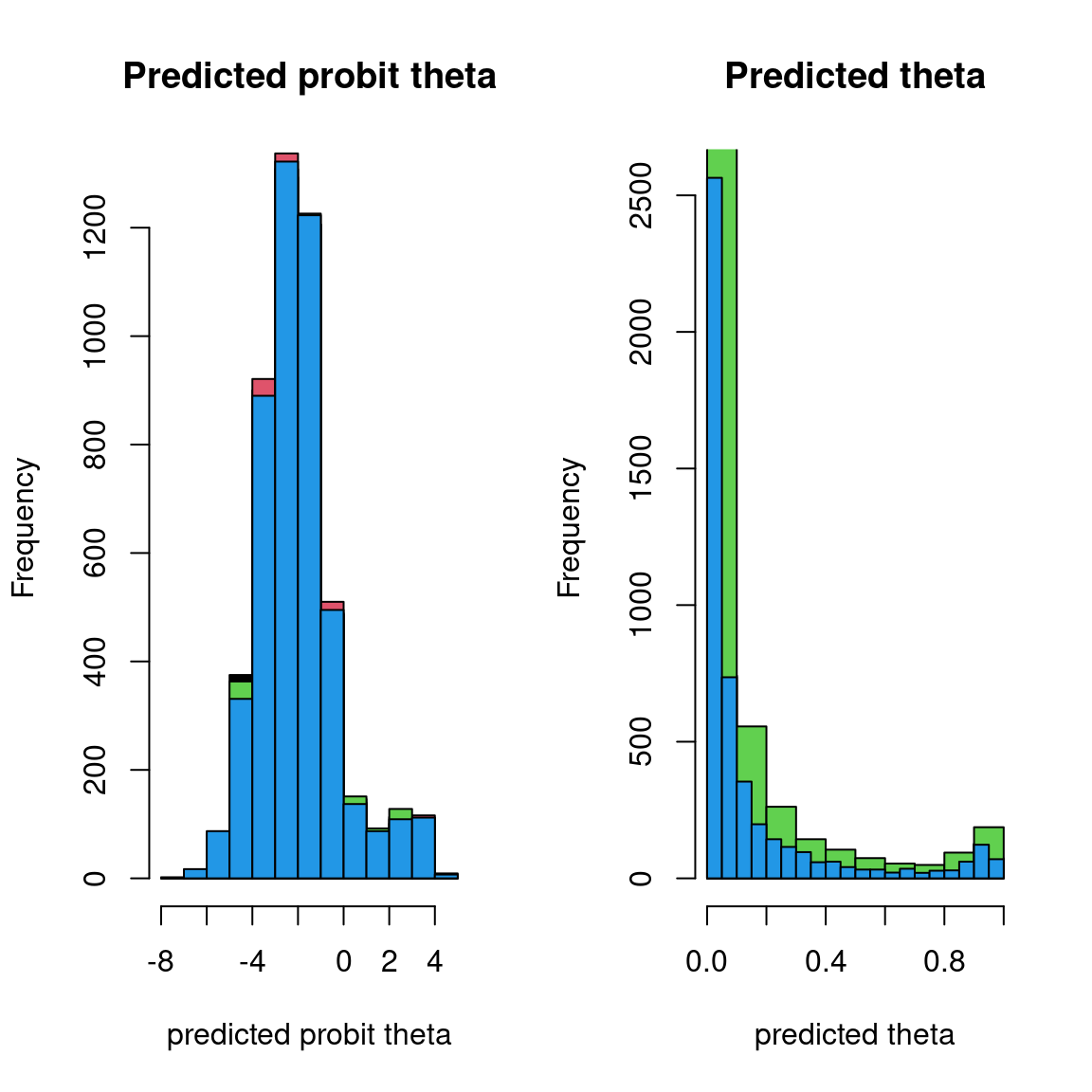
Overall, the traces and the densities of the parameters indicate the convergence of the algorithm. Indeed, we observe on the traces that the values oscillate around averages without showing an upward or downward trend and we see that the densities are quite smooth and for the most part of Gaussian form, even for the factor loadings \(\lambda\) constrained to be positive.
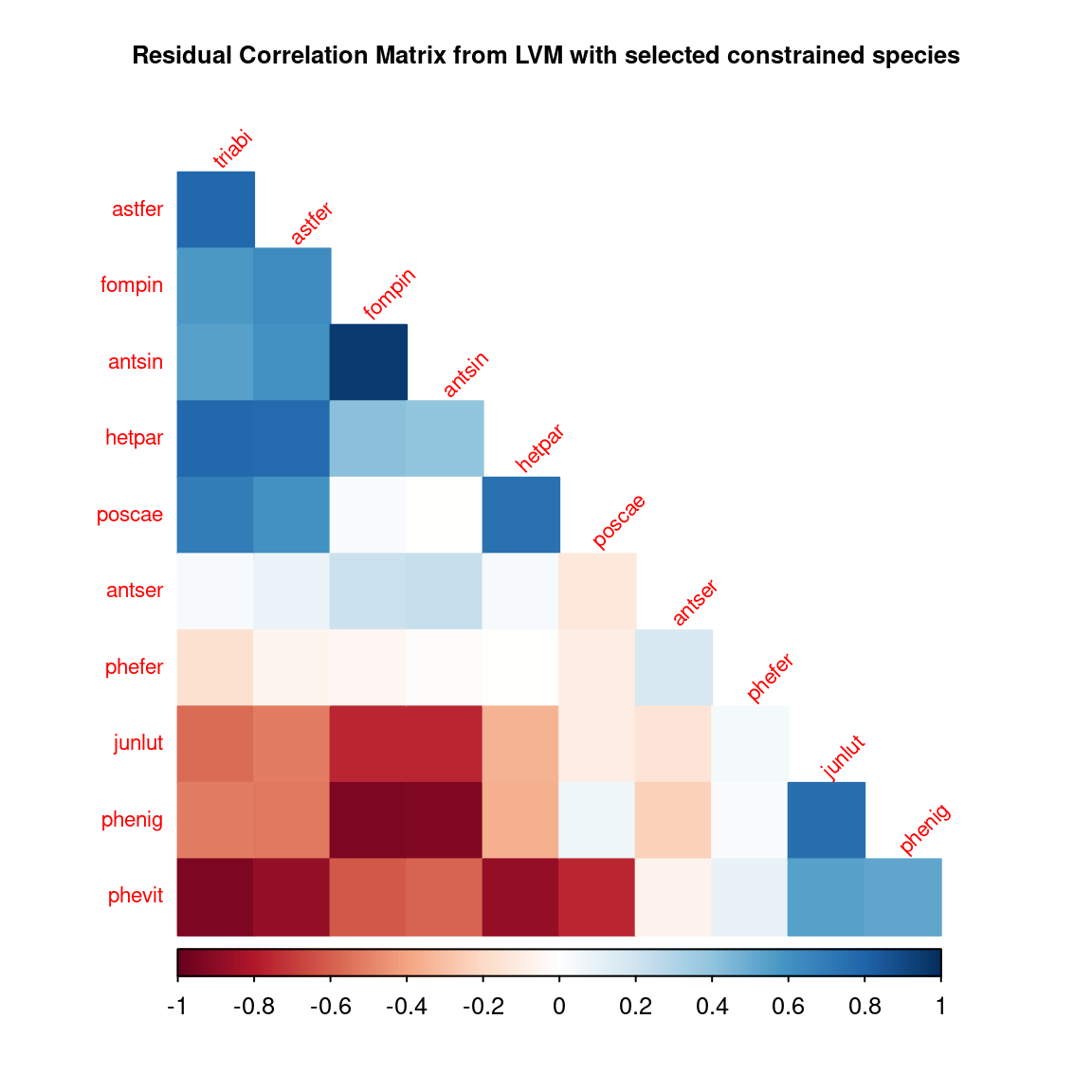
5.3.2 Matrice of residual correlations
After fitting the jSDM with latent variables, the full species residual correlation matrix \(R=(R_{ij})^{i=1,\ldots, nspecies}_{j=1,\ldots, nspecies}\) can be derived from the covariance in the latent variables such as : \[\Sigma_{ij} = \lambda_i .\lambda_j^T\], then we compute correlations from covariances : \[R_{i,j} = \frac{\Sigma_{ij}}{\sqrt{\Sigma _{ii}\Sigma _{jj}}}\].
We use the get_residual_cor() function to compute the residual correlation matrix \(R\) for each chain and we display the average of the \(R\) matrices for all chains:
nsp <- ncol(mod_Fungi_ord[[1]]$model_spec$beta_start)
# Average residual correlation matrix on the four mcmc chains
R <- matrix(0, nsp, nsp)
for(i in 1:nchains){
R <- R + get_residual_cor(mod_Fungi_ord[[i]])$cor.mean/nchains
}
colnames(R) <- rownames(R) <- colnames(PA_Fungi)
R.reorder <- corrplot::corrMatOrder(R, order = "FPC", hclust.method = "average")
corrplot::corrplot(R[R.reorder,R.reorder], diag = F, type = "lower", cex.main=0.8,
method = "color", mar = c(1,1,3,1),
tl.srt = 45, tl.cex = 0.7,
title = "Residual Correlation Matrix from LVM with selected constrained species")